Five-Year Survival Outcomes of Laparoscopy-Assisted vs Open Distal Gastrectomy for Advanced Gastric Cancer: The JLSSG0901 Randomized Clinical Trial
- PMID: 36920382
- PMCID: PMC10018406
- DOI: 10.1001/jamasurg.2023.0096
Five-Year Survival Outcomes of Laparoscopy-Assisted vs Open Distal Gastrectomy for Advanced Gastric Cancer: The JLSSG0901 Randomized Clinical Trial
Abstract
Importance: Evidence of implementation of laparoscopic gastrectomy for locally advanced gastric cancer is currently insufficient, as the primary end point in previous prospective studies was evaluated at a median follow-up time of 3 years. More robust evidence is necessary to verify noninferiority of laparoscopic gastrectomy.
Objective: To compare 5-year survival outcomes between laparoscopy-assisted distal gastrectomy (LADG) and open distal gastrectomy (ODG) with D2 lymph node dissection for locally advanced gastric cancer.
Design, setting, and participants: This was a multicenter, open-label, noninferiority, prospective randomized clinical trial. Between November 26, 2009, and July 29, 2016, eligible patients with histologically proven gastric carcinoma from 37 institutes in Japan were enrolled. Two interim analyses and final analysis were performed in October 2014, May 2018, and November 2021, respectively.
Interventions: Patients were randomly assigned (1:1) to either the ODG or LADG group. The procedures were performed exclusively by qualified surgeons.
Main outcomes and measures: The primary end point was 5-year relapse-free survival, and the noninferiority margin for the hazard ratio (HR) was set at 1.31. The secondary end points were 5-year overall survival and safety.
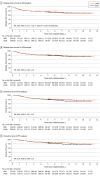
Results: A total of 502 patients were included in the full-analysis set: 254 (50.6%) in the ODG group and 248 (49.4%) in the LADG group. Patients in the ODG group had a median (IQR) age of 67 (33-80) years and included 168 males (66.1%). Patients in the LADG group had a median (IQR) age of 64 (34-80) years and included 169 males (68.1%). No significant differences were observed in severe postoperative complications between the 2 groups in the safety analysis (ODG, 4.7% [11 of 233] vs LADG, 3.5% [8 of 227]; P = .64). The median (IQR) follow-up for all patients after randomization was 67.9 (60.3-92.0) months. The 5-year relapse-free survival was 73.9% (95% CI, 68.7%-79.5%) and 75.7% (95% CI, 70.5%-81.2%) for the ODG and LADG groups, respectively, and the HR was 0.96 (90% CI, 0.72-1.26; noninferiority 1-sided P = .03). Further, no significant difference was observed in overall survival time between the 2 groups, and the HR was 0.83 (95% CI, 0.57-1.21; P = .34). The pattern of recurrence was similar between the 2 groups.
Conclusions and relevance: Results of this study show that on the basis of 5-year follow-up data, LADG with D2 lymph node dissection for locally advanced gastric cancer, when performed by qualified surgeons, was proved noninferior to ODG. This laparoscopic approach could become a standard treatment for locally advanced gastric cancer.
Trial registration: UMIN Clinical Trial Registry: UMIN000003420.
Conflict of interest statement
Figures


Comment in
-
Laparoscopic Gastrectomy for Gastric Cancer.JAMA Surg. 2023 May 1;158(5):454-455. doi: 10.1001/jamasurg.2023.0109. JAMA Surg. 2023. PMID: 36920402 No abstract available.
References
-
- Kitano S, Iso Y, Moriyama M, Sugimachi K. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc. 1994;4(2):146-148. - PubMed
-
- Katai H, Mizusawa J, Katayama H, et al. Short-term surgical outcomes from a phase III study of laparoscopy-assisted versus open distal gastrectomy with nodal dissection for clinical stage IA/IB gastric cancer: Japan Clinical Oncology Group Study JCOG0912. Gastric Cancer. 2017;20(4):699-708. doi: 10.1007/s10120-016-0646-9 - DOI - PubMed
-
- Kim HH, Han SU, Kim MC, et al. ; Korean Laparoendoscopic Gastrointestinal Surgery Study (KLASS) Group . Effect of laparoscopic distal gastrectomy vs open distal gastrectomy on long-term survival among patients with stage I gastric cancer: the KLASS-01 randomized clinical trial. JAMA Oncol. 2019;5(4):506-513. doi: 10.1001/jamaoncol.2018.6727 - DOI - PMC - PubMed
-
- Katai H, Mizusawa J, Katayama H, et al. Survival outcomes after laparoscopy-assisted distal gastrectomy versus open distal gastrectomy with nodal dissection for clinical stage IA or IB gastric cancer (JCOG0912): a multicentre, non-inferiority, phase 3 randomised controlled trial. Lancet Gastroenterol Hepatol. 2020;5(2):142-151. doi: 10.1016/S2468-1253(19)30332-2 - DOI - PubMed
Publication types
MeSH terms
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Medical

