Deep learning for automated, interpretable classification of lumbar spinal stenosis and facet arthropathy from axial MRI
- PMID: 36920520
- PMCID: PMC10566647
- DOI: 10.1007/s00330-023-09483-6
Deep learning for automated, interpretable classification of lumbar spinal stenosis and facet arthropathy from axial MRI
Abstract
Objectives: To evaluate a deep learning model for automated and interpretable classification of central canal stenosis, neural foraminal stenosis, and facet arthropathy from lumbar spine MRI.
Methods: T2-weighted axial MRI studies of the lumbar spine acquired between 2008 and 2019 were retrospectively selected (n = 200) and graded for central canal stenosis, neural foraminal stenosis, and facet arthropathy. Studies were partitioned into patient-level train (n = 150), validation (n = 20), and test (n = 30) splits. V-Net models were first trained to segment the dural sac and the intervertebral disk, and localize facet and foramen using geometric rules. Subsequently, Big Transfer (BiT) models were trained for downstream classification tasks. An interpretable model for central canal stenosis was also trained using a decision tree classifier. Evaluation metrics included linearly weighted Cohen's kappa score for multi-grade classification and area under the receiver operator characteristic curve (AUROC) for binarized classification.
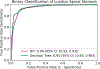
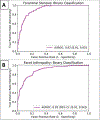
Results: Segmentation of the dural sac and intervertebral disk achieved Dice scores of 0.93 and 0.94. Localization of foramen and facet achieved intersection over union of 0.72 and 0.83. Multi-class grading of central canal stenosis achieved a kappa score of 0.54. The interpretable decision tree classifier had a kappa score of 0.80. Pairwise agreement between readers (R1, R2), (R1, R3), and (R2, R3) was 0.86, 0.80, and 0.74. Binary classification of neural foraminal stenosis and facet arthropathy achieved AUROCs of 0.92 and 0.93.
Conclusion: Deep learning systems can be performant as well as interpretable for automated evaluation of lumbar spine MRI including classification of central canal stenosis, neural foraminal stenosis, and facet arthropathy.
Key points: • Interpretable deep-learning systems can be developed for the evaluation of clinical lumbar spine MRI. Multi-grade classification of central canal stenosis with a kappa of 0.80 was comparable to inter-reader agreement scores (0.74, 0.80, 0.86). Binary classification of neural foraminal stenosis and facet arthropathy achieved favorable and accurate AUROCs of 0.92 and 0.93, respectively. • While existing deep-learning systems are opaque, leading to clinical deployment challenges, the proposed system is accurate as well as interpretable, providing valuable information to a radiologist in clinical practice.
Keywords: Arthropathy; Deep learning; MRI, Stenosis.
© 2023. The Author(s), under exclusive licence to European Society of Radiology.
Conflict of interest statement
Figures




References
-
- Deyo RA, Gray D, Kreuter W, Mirza S, Martin BI (2005) United States trends in lumbar fusion surgery for degenerative conditions. (Phila Pa 1976) 30:1441–1445 - PubMed
-
- Cowley P (2016) Neuroimaging of spinal canal stenosis. Magn Reson Imaging Clin N Am 24:523–529 - PubMed
-
- Morita M, Miyauchi A, Okuda S, Oda T, Iwasaki M (2011) Comparison between MRI and myelography in lumbar spinal canal stenosis for the decision of levels of decompression surgery. J Spinal Disord Tech 24:31–36 - PubMed
-
- Alsaleh K, Ho D, Rosas-Arellano MP, Stewart TC, Gurr KR, Bailey CS (2017) Radiographic assessment of degenerative lumbar spinal stenosis: is MRI superior to CT? Eur Spine J 26:362–367 - PubMed

