Clinical Practice Guidelines for Pancreatic Cancer 2022 from the Japan Pancreas Society: a synopsis
- PMID: 36920680
- PMCID: PMC10066137
- DOI: 10.1007/s10147-023-02317-x
Clinical Practice Guidelines for Pancreatic Cancer 2022 from the Japan Pancreas Society: a synopsis
Abstract
Objectives: Clinical Practice Guidelines for Pancreatic Cancer was first published in 2006 by the Japan Pancreas Society, and revised in 2009, 2013, 2016, and 2019. In July 2022, Clinical Practice Guidelines for Pancreatic Cancer was newly revised in Japanese.
Methods: For this revision, we developed an entirely new guideline according to the Minds Manual for Guideline Development 2020, which includes the concepts of GRADE-Grading Recommendations Assessment, Development, and Evaluation, to enable a better understanding of the current guidelines. Patients and the public were actively involved in both the development and implementation of the guideline.
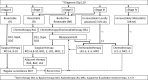
Results: The guideline includes algorithms for diagnosis, treatment, chemotherapy, and precision medicine of pancreatic cancer, and addresses 7 subjects: diagnosis, surgical therapy, adjuvant therapy, radiation therapy, chemotherapy, stent therapy, and supportive & palliative medical care. It includes 73 clinical questions and 112 statements. The statements correspond to the clinical questions, evidence levels, recommendation strengths, and agreement rates.
Conclusions: This guideline represents the most standard clinical and practical management guideline available until date in Japan. This is the English synopsis of the Clinical Practice Guidelines for Pancreatic Cancer 2022 in Japanese, and is an attempt to disseminate the Japanese guideline worldwide to introduce the Japanese approach to the clinical management of pancreatic cancer.
Keywords: Clinical guidelines; GRADE system; Japan Pancreas Society; Minds; Pancreatic cancer.
© 2023. The Author(s).
Conflict of interest statement
Members of the Guideline Review Committee behaved in accordance with the Japanese Society of Clinical Oncology (JSCO) Guidelines for Conflict of Interest Issues Related to Clinical Studies of Oncology. Potential conflicts of interest of individual committee members are declared at Development of these guidelines was funded and supported by the Japan Pancreas Society (President, Yoshifumi Takeyama). Neither the opinions nor the interests of the funding body affected the final guideline recommendations.
Figures



References
-
- Japan Pancreas Society . Evidence-based medicine clinical practice guidelines for pancreatic cancer 2006. Tokyo: Kanahara & Co Ltd; 2006.
-
- Japan Pancreas Society . Evidence-Based Medicine Clinical Practice Guidelines for Pancreatic Cancer. Tokyo: Kanahara & Co, Ltd.; 2009.
-
- Japan Pancreas Society . Evidence-Based Medicine Clinical Practice Guidelines for Pancreatic Cancer 2013. Tokyo: Kanahara & Co, Ltd.; 2013.
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

