C1-inhibitor treatment in patients with severe complement-mediated autoimmune hemolytic anemia
- PMID: 36920779
- PMCID: PMC10362545
- DOI: 10.1182/bloodadvances.2022009402
C1-inhibitor treatment in patients with severe complement-mediated autoimmune hemolytic anemia
Abstract
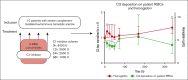
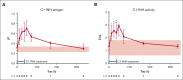
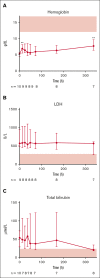
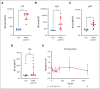
Complement-mediated (CM) autoimmune hemolytic anemia (AIHA) is characterized by the destruction of red blood cells (RBCs) by autoantibodies that activate the classical complement pathway. These antibodies also reduce transfusion efficacy via the lysis of donor RBCs. Because C1-inhibitor (C1-INH) is an endogenous regulator of the classical complement pathway, we hypothesized that peritransfusional C1-INH in patients with severe CM-AIHA reduces complement activation and hemolysis, and thus enhances RBC transfusion efficacy. We conducted a prospective, single-center, phase 2, open-label trial (EudraCT2012-003710-13). Patients with confirmed CM-AIHA and indication for the transfusion of 2 RBC units were eligible for inclusion. Four IV C1-INH doses (6000, 3000, 2000, and 1000 U) were administered with 12-hour intervals around RBC transfusion. Serial blood samples were analyzed for hemolytic activity, RBC opsonization, complement activation, and inflammation markers. Ten patients were included in the study. C1-INH administration increased plasma C1-INH antigen and activity, peaking at 48 hours after the first dose and accompanied by a significant reduction of RBC C3d deposition. Hemoglobin levels increased briefly after transfusion but returned to baseline within 48 hours. Overall, markers of hemolysis, inflammation, and complement activation remained unchanged. Five grade 3 and 1 grade 4 adverse event occurred but were considered unrelated to the study medication. In conclusion, peritransfusional C1-INH temporarily reduced complement activation. However, C1-INH failed to halt hemolytic activity in severe transfusion-dependent-CM-AIHA. We cannot exclude that posttransfusional hemolytic activity would have been even higher without C1-INH. The potential of complement inhibition on transfusion efficacy in severe CM-AIHA remains to be determined.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: M.J.K. reports honoraria, consultancy, and advisory for Kite, Novartis, Miltenyi Biotech, Roche, and Bristol Myers Squibb (BMS)/Celgene; research funding from Kite, Roche, Takeda, and Celgene; and travel support from Kite, Roche, Novartis, and Miltenyi Biotech; all honoraria are institutional. S.Z. reports honoraria and speaker fees from Sobi, Roche, Alexion, Sanofi, and Jazz and unrestricted grants from Jazz. J.M.I.V. reports consultancy and advisory for Sanofi; research funding from Beigene; and conference support from BMS; all honoraria are institutional. The remaining authors declare no competing financial interests.
The current affiliation for E.M.M. is Janssen Vaccines & Prevention B.V., Leiden, The Netherlands.
Figures



References
-
- Berentsen S. How I treat cold agglutinin disease. Blood. 2021;137(10):1295–1303. - PubMed
-
- Jäger U, Barcellini W, Broome CM, et al. Diagnosis and treatment of autoimmune hemolytic anemia in adults: recommendations from the first International Consensus Meeting. Blood Rev. 2020;41 - PubMed
-
- Barcellini W, Fattizzo B, Zaninoni A, et al. Clinical heterogeneity and predictors of outcome in primary autoimmune hemolytic anemia: a GIMEMA study of 308 patients. Blood. 2014;124(19):2930–2936. - PubMed

