The mitochondrial pyruvate carrier complex potentiates the efficacy of proteasome inhibitors in multiple myeloma
- PMID: 36920785
- PMCID: PMC10362273
- DOI: 10.1182/bloodadvances.2022008345
The mitochondrial pyruvate carrier complex potentiates the efficacy of proteasome inhibitors in multiple myeloma
Abstract
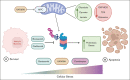
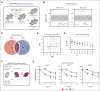
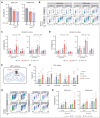
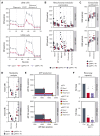
Multiple myeloma (MM) is a hematological malignancy that emerges from antibody-producing plasma B cells. Proteasome inhibitors, including the US Food and Drug Administration-approved bortezomib (BTZ) and carfilzomib (CFZ), are frequently used for the treatment of patients with MM. Nevertheless, a significant proportion of patients with MM are refractory or develop resistance to this class of inhibitors, which represents a significant challenge in the clinic. Thus, identifying factors that determine the potency of proteasome inhibitors in MM is of paramount importance to bolster their efficacy in the clinic. Using genome-wide CRISPR-based screening, we identified a subunit of the mitochondrial pyruvate carrier (MPC) complex, MPC1, as a common modulator of BTZ response in 2 distinct human MM cell lines in vitro. We noticed that CRISPR-mediated deletion or pharmacological inhibition of the MPC complex enhanced BTZ/CFZ-induced MM cell death with minimal impact on cell cycle progression. In fact, targeting the MPC complex compromised the bioenergetic capacity of MM cells, which is accompanied by reduced proteasomal activity, thereby exacerbating BTZ-induced cytotoxicity in vitro. Importantly, we observed that the RNA expression levels of several regulators of pyruvate metabolism were altered in advanced stages of MM for which they correlated with poor patient prognosis. Collectively, this study highlights the importance of the MPC complex for the survival of MM cells and their responses to proteasome inhibitors. These findings establish mitochondrial pyruvate metabolism as a potential target for the treatment of MM and an unappreciated strategy to increase the efficacy of proteasome inhibitors in the clinic.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures

References
-
- Bazarbachi AH, Al Hamed R, Malard F, Harousseau JL, Mohty M. Relapsed refractory multiple myeloma: a comprehensive overview. Leukemia. 2019;33(10):2343–2357. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

