Circulating SARS-CoV-2+ megakaryocytes are associated with severe viral infection in COVID-19
- PMID: 36920790
- PMCID: PMC10022176
- DOI: 10.1182/bloodadvances.2022009022
Circulating SARS-CoV-2+ megakaryocytes are associated with severe viral infection in COVID-19
Abstract
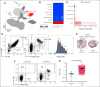
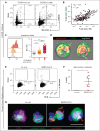
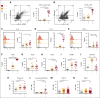
Several independent lines of evidence suggest that megakaryocytes are dysfunctional in severe COVID-19. Herein, we characterized peripheral circulating megakaryocytes in a large cohort of inpatients with COVID-19 and correlated the subpopulation frequencies with clinical outcomes. Using peripheral blood, we show that megakaryocytes are increased in the systemic circulation in COVID-19, and we identify and validate S100A8/A9 as a defining marker of megakaryocyte dysfunction. We further reveal a subpopulation of S100A8/A9+ megakaryocytes that contain severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) protein and RNA. Using flow cytometry of peripheral blood and in vitro studies on SARS-CoV-2-infected primary human megakaryocytes, we demonstrate that megakaryocytes can transfer viral antigens to emerging platelets. Mechanistically, we show that SARS-CoV-2-containing megakaryocytes are nuclear factor κB (NF-κB)-activated, via p65 and p52; express the NF-κB-mediated cytokines interleukin-6 (IL-6) and IL-1β; and display high surface expression of Toll-like receptor 2 (TLR2) and TLR4, canonical drivers of NF-κB. In a cohort of 218 inpatients with COVID-19, we correlate frequencies of megakaryocyte subpopulations with clinical outcomes and show that SARS-CoV-2-containing megakaryocytes are a strong risk factor for mortality and multiorgan injury, including respiratory failure, mechanical ventilation, acute kidney injury, thrombotic events, and intensive care unit admission. Furthermore, we show that SARS-CoV-2+ megakaryocytes are present in lung and brain autopsy tissues from deceased donors who had COVID-19. To our knowledge, this study offers the first evidence implicating SARS-CoV-2+ peripheral megakaryocytes in severe disease and suggests that circulating megakaryocytes warrant investigation in inflammatory disorders beyond COVID-19.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures




Comment in
-
Peripheral megakaryocytes sound the alarm in COVID-19.Blood Adv. 2023 Aug 8;7(15):4197-4199. doi: 10.1182/bloodadvances.2023010201. Blood Adv. 2023. PMID: 37552512 Free PMC article. No abstract available.
References
-
- Tavassoli M, Aoki M. Migration of entire megakaryocytes through the marrow--blood barrier. Br J Haematol. 1981;48(1):25–29. - PubMed
-
- Woods MJ, Greaves M, Smith GH, Trowbridge EA. The fate of circulating megakaryocytes during cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1993;106(4):658–663. - PubMed
-
- Dejima H, Nakanishi H, Kuroda H, et al. Detection of abundant megakaryocytes in pulmonary artery blood in lung cancer patients using a microfluidic platform. Lung Cancer. 2018;125:128–135. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

