Dopamine transporter and synaptic vesicle sorting defects underlie auxilin-associated Parkinson's disease
- PMID: 36920906
- PMCID: PMC10127800
- DOI: 10.1016/j.celrep.2023.112231
Dopamine transporter and synaptic vesicle sorting defects underlie auxilin-associated Parkinson's disease
Abstract
Auxilin participates in the uncoating of clathrin-coated vesicles (CCVs), thereby facilitating synaptic vesicle (SV) regeneration at presynaptic sites. Auxilin (DNAJC6/PARK19) loss-of-function mutations cause early-onset Parkinson's disease (PD). Here, we utilized auxilin knockout (KO) mice to elucidate the mechanisms through which auxilin deficiency and clathrin-uncoating deficits lead to PD. Auxilin KO mice display cardinal features of PD, including progressive motor deficits, α-synuclein pathology, nigral dopaminergic loss, and neuroinflammation. Significantly, treatment with L-DOPA ameliorated motor deficits. Unbiased proteomic and neurochemical analyses of auxilin KO brains indicated dopamine dyshomeostasis. We validated these findings by demonstrating slower dopamine reuptake kinetics in vivo, an effect associated with dopamine transporter misrouting into axonal membrane deformities in the dorsal striatum. Defective SV protein sorting and elevated synaptic autophagy also contribute to ineffective dopamine sequestration and compartmentalization, ultimately leading to neurodegeneration. This study provides insights into how presynaptic endocytosis deficits lead to dopaminergic vulnerability and pathogenesis of PD.
Keywords: CP: Neuroscience; L-DOPA; Lewy bodies; axonal deformity; clathrin-coated vesicles; clathrin-mediated endocytosis; dopamine; dorsal striatum; endolysosomal system; substantia nigra; synaptic autophagy; ∝-synuclein.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures
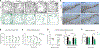
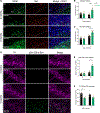


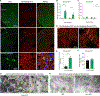

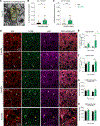
References
-
- Edvardson S, Cinnamon Y, Ta-Shma A, Shaag A, Yim YI, Zenvirt S, Jalas C, Lesage S, Brice A, Taraboulos A, et al. (2012). A deleterious mutation in DNAJC6 encoding the neuronal-specific clathrin-uncoating co-chaperone auxilin, is associated with juvenile parkinsonism. PLoS One 7, e36458. 10.1371/journal.pone.0036458. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

