Autolysin-mediated peptidoglycan hydrolysis is required for the surface display of Staphylococcus aureus cell wall-anchored proteins
- PMID: 36920922
- PMCID: PMC10041135
- DOI: 10.1073/pnas.2301414120
Autolysin-mediated peptidoglycan hydrolysis is required for the surface display of Staphylococcus aureus cell wall-anchored proteins
Abstract
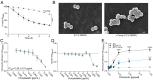
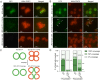
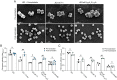
Peptidoglycan hydrolases, or autolysins, play a critical role in cell wall remodeling and degradation, facilitating bacterial growth, cell division, and cell separation. In Staphylococcus aureus, the so-called "major" autolysin, Atl, has long been associated with host adhesion; however, the molecular basis underlying this phenomenon remains understudied. To investigate, we used the type V glycopeptide antibiotic complestatin, which binds to peptidoglycan and blocks the activity of autolysins, as a chemical probe of autolysin function. We also generated a chromosomally encoded, catalytically inactive variant of the Atl enzyme. Autolysin-mediated peptidoglycan hydrolysis, in particular Atl-mediated daughter cell separation, was shown to be critical for maintaining optimal surface levels of S. aureus cell wall-anchored proteins, including the fibronectin-binding proteins (FnBPs) and protein A (Spa). As such, disrupting autolysin function reduced the affinity of S. aureus for host cell ligands, and negatively impacted early stages of bacterial colonization in a systemic model of S. aureus infection. Phenotypic studies revealed that Spa was sequestered at the septum of complestatin-treated cells, highlighting that autolysins are required to liberate Spa during cell division. In summary, we reveal the hydrolytic activities of autolysins are associated with the surface display of S. aureus cell wall-anchored proteins. We demonstrate that by blocking autolysin function, type V glycopeptide antibiotics are promising antivirulence agents for the development of strategies to control S. aureus infections.
Keywords: Staphylococcus aureus; antibiotic resistance; autolysins; host adhesion; virulence.
Conflict of interest statement
The authors declare no competing interest.
Figures



References
-
- Berry K. A., Verhoef M. T. A., Leonard A. C., Cox G., Staphylococcus aureus adhesion to the host. Ann. N. Y. Acad. Sci. 1515, 75–96 (2022). - PubMed
-
- Leonard A. C., Petrie L. E., Cox G., Bacterial anti-adhesives: Inhibition of Staphylococcus aureus nasal colonization. ACS Infect. Dis. 5, 1668–1681 (2019). - PubMed
-
- Biswas R., et al. , Activity of the major staphylococcal autolysin Atl. FEMS Microbiol. Lett. 259, 260–268 (2006). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

