Building insightful, memory-enriched models to capture long-time biochemical processes from short-time simulations
- PMID: 36920924
- PMCID: PMC10041170
- DOI: 10.1073/pnas.2221048120
Building insightful, memory-enriched models to capture long-time biochemical processes from short-time simulations
Abstract
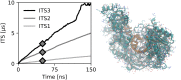
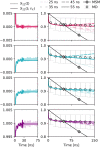
The ability to predict and understand complex molecular motions occurring over diverse timescales ranging from picoseconds to seconds and even hours in biological systems remains one of the largest challenges to chemical theory. Markov state models (MSMs), which provide a memoryless description of the transitions between different states of a biochemical system, have provided numerous important physically transparent insights into biological function. However, constructing these models often necessitates performing extremely long molecular simulations to converge the rates. Here, we show that by incorporating memory via the time-convolutionless generalized master equation (TCL-GME) one can build a theoretically transparent and physically intuitive memory-enriched model of biochemical processes with up to a three order of magnitude reduction in the simulation data required while also providing a higher temporal resolution. We derive the conditions under which the TCL-GME provides a more efficient means to capture slow dynamics than MSMs and rigorously prove when the two provide equally valid and efficient descriptions of the slow configurational dynamics. We further introduce a simple averaging procedure that enables our TCL-GME approach to quickly converge and accurately predict long-time dynamics even when parameterized with noisy reference data arising from short trajectories. We illustrate the advantages of the TCL-GME using alanine dipeptide, the human argonaute complex, and FiP35 WW domain.
Keywords: Markov state models; biomolecular dynamics; generalized master equations; memory effects; protein folding.
Conflict of interest statement
The authors declare no competing interest.
Figures


References
-
- Chaudhuri T. K., Paul S., Protein-misfolding diseases and chaperone-based therapeutic approaches. FEBS J. 273, 1331 (2006). - PubMed
-
- Wang W., Cao S., Zhu L., Huang X., Constructing Markov state models to elucidate the functional conformational changes of complex biomolecules. WIREs Comput. Mol. Sci. 8 (2018).
-
- Husic B. E., Pande V. S., Markov state models: From an art to a science. J. Am. Chem. Soc. 140, 2386 (2018). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

