Preclinical evaluation of Insulin-like growth factor receptor 1 (IGF1R) and Insulin Receptor (IR) as a therapeutic targets in triple negative breast cancer
- PMID: 36920947
- PMCID: PMC10016661
- DOI: 10.1371/journal.pone.0282512
Preclinical evaluation of Insulin-like growth factor receptor 1 (IGF1R) and Insulin Receptor (IR) as a therapeutic targets in triple negative breast cancer
Abstract
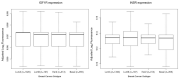
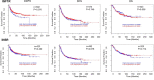
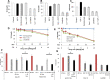
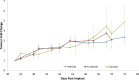
Triple Negative Breast Cancer (TNBC), a subtype of breast cancer, has fewer successful therapeutic therapies than other types of breast cancer. Insulin-like growth factor receptor 1 (IGF1R) and the Insulin receptor (IR) are associated with poor outcomes in TNBC. Targeting IGF1R has failed clinically. We aimed to test if inhibiting both IR/IGF1R was a rationale therapeutic approach to treat TNBC. We showed that despite IGF1R and IR being expressed in TNBC, their expression is not associated with a negative survival outcome. Furthermore, targeting both IR/IGF1R with inhibitors in multiple TNBC cell lines did not inhibit cell growth. Linsitinib, a small molecule inhibitor of both IGF1R and IR, did not block tumour formation and had no effect on tumour growth in vivo. Cumulatively these data suggest that while IGF1R and IR are expressed in TNBC, they are not good therapeutic targets. A potential reason for the limited anti-cancer impact when IR/IGF1R was targeted may be because multiple signalling pathways are altered in TNBC. Therefore, targeting individual signalling pathways may not be sufficient to inhibit cancer growth.
Copyright: © 2023 Roche et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Dolgin E. Atezolizumab Combo Approved for PD-L1–positive TNBC. Cancer Discov. 2019;9: OF2. doi: 10.1158/2159-8290.CD-NB2019-038 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

