The BAF complex inhibitor pyrimethamine reverses HIV-1 latency in people with HIV-1 on antiretroviral therapy
- PMID: 36921041
- PMCID: PMC10017042
- DOI: 10.1126/sciadv.ade6675
The BAF complex inhibitor pyrimethamine reverses HIV-1 latency in people with HIV-1 on antiretroviral therapy
Abstract
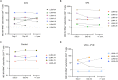
Reactivation of the latent HIV-1 reservoir is a first step toward triggering reservoir decay. Here, we investigated the impact of the BAF complex inhibitor pyrimethamine on the reservoir of people living with HIV-1 (PLWH). Twenty-eight PLWH on suppressive antiretroviral therapy were randomized (1:1:1:1 ratio) to receive pyrimethamine, valproic acid, both, or no intervention for 14 days. The primary end point was change in cell-associated unspliced (CA US) HIV-1 RNA at days 0 and 14. We observed a rapid, modest, and significant increase in (CA US) HIV-1 RNA in response to pyrimethamine exposure, which persisted throughout treatment and follow-up. Valproic acid treatment alone did not increase (CA US) HIV-1 RNA or augment the effect of pyrimethamine. Pyrimethamine treatment did not result in a reduction in the size of the inducible reservoir. These data demonstrate that the licensed drug pyrimethamine can be repurposed as a BAF complex inhibitor to reverse HIV-1 latency in vivo in PLWH, substantiating its potential advancement in clinical studies.
Figures




References
-
- Chun T. W., Davey R. T. Jr., Ostrowski M., Shawn Justement J., Engel D., Mullins J. I., Fauci A. S., Relationship between pre-existing viral reservoirs and the re-emergence of plasma viremia after discontinuation of highly active anti-retroviral therapy. Nat. Med. 6, 757–761 (2000). - PubMed
-
- Strategies for Management of Antiretroviral Therapy (SMART) Study Group, El-Sadr W. M., Lundgren J., Neaton J. D., Gordin F., Abrams D., Arduino R. C., Babiker A., Burman W., Clumeck N., Cohen C. J., Cohn D., Cooper D., Darbyshire J., Emery S., Fatkenheuer G., Gazzard B., Grund B., Hoy J., Klingman K., Losso M., Markowitz N., Neuhaus J., Phillips A., Rappoport C., CD4+ count–guided interruption of antiretroviral treatment. N. Engl. J. Med. 355, 2283–2296 (2006). - PubMed
-
- Deeks S. G., Shock and kill. Nature 487, 439–440 (2012). - PubMed
-
- Deeks S. G., Archin N., Cannon P., Collins S., Jones R. B., de Jong M., Lambotte O., Lamplough R., Ndung'u T., Sugarman J., Tiemessen C. T., Vandekerckhove L., Lewin S. R.; The International AIDS Society (IAS) Global Scientific Strategy working group , Research priorities for an HIV cure: International AIDS Society Global Scientific Strategy 2021. Nat. Med. 27, 2085–2098 (2021). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

