Volume-transmitted GABA waves pace epileptiform rhythms in the hippocampal network
- PMID: 36921605
- PMCID: PMC10615848
- DOI: 10.1016/j.cub.2023.02.051
Volume-transmitted GABA waves pace epileptiform rhythms in the hippocampal network
Abstract
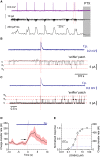
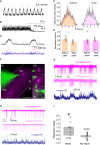
Mechanisms that entrain and pace rhythmic epileptiform discharges remain debated. Traditionally, the quest to understand them has focused on interneuronal networks driven by synaptic GABAergic connections. However, synchronized interneuronal discharges could also trigger the transient elevations of extracellular GABA across the tissue volume, thus raising tonic conductance (Gtonic) of synaptic and extrasynaptic GABA receptors in multiple cells. Here, we monitor extracellular GABA in hippocampal slices using patch-clamp GABA "sniffer" and a novel optical GABA sensor, showing that periodic epileptiform discharges are preceded by transient, region-wide waves of extracellular GABA. Neural network simulations that incorporate volume-transmitted GABA signals point to a cycle of GABA-driven network inhibition and disinhibition underpinning this relationship. We test and validate this hypothesis using simultaneous patch-clamp recordings from multiple neurons and selective optogenetic stimulation of fast-spiking interneurons. Critically, reducing GABA uptake in order to decelerate extracellular GABA fluctuations-without affecting synaptic GABAergic transmission or resting GABA levels-slows down rhythmic activity. Our findings thus unveil a key role of extrasynaptic, volume-transmitted GABA in pacing regenerative rhythmic activity in brain networks.
Keywords: GABA uptake; GAT-1; brain rhythms; epilepsy; extracellular GABA; iGABASnFR2; spiking neural networks; tonic GABA conductance; volume transmission.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures





References
-
- Ben-Ari Y., Gaiarsa J.L., Tyzio R., Khazipov R. GABA: a pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiol. Rev. 2007;87:1215–1284. - PubMed
-
- van der Zeyden M., Oldenziel W.H., Rea K., Cremers T.I., Westerink B.H. Microdialysis of GABA and glutamate: analysis, interpretation and comparison with microsensors. Pharmacol. Biochem. Behav. 2008;90:135–147. - PubMed
-
- Scanziani M. GABA spillover activates postsynaptic GABA(B) receptors to control rhythmic hippocampal activity. Neuron. 2000;25:673–681. - PubMed
-
- Loo D.D.F., Eskandari S., Boorer K.J., Sarkar H.K., Wright E.M. Role of Cl- in electrogenic Na+-coupled cotransporters GAT1 and SGLT1. J. Biol. Chem. 2000;275:37414–37422. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- MR/V034758/1/MRC_/Medical Research Council/United Kingdom
- G0400136/MRC_/Medical Research Council/United Kingdom
- 212251/Z/18/Z /WT_/Wellcome Trust/United Kingdom
- MR/V013556/1/MRC_/Medical Research Council/United Kingdom
- MRF-007-0004-STD-KULLM/MRF_/MRF_/United Kingdom
- MR/W019752/1/MRC_/Medical Research Council/United Kingdom
- 212285/Z/18/Z/WT_/Wellcome Trust/United Kingdom
- G0400627/MRC_/Medical Research Council/United Kingdom
- MR/W005204/1/MRC_/Medical Research Council/United Kingdom
- G0801316/MRC_/Medical Research Council/United Kingdom
- 323113/ERC_/European Research Council/International
- 209807/Z/17/Z/WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

