Lactate regulates cell cycle by remodelling the anaphase promoting complex
- PMID: 36921622
- PMCID: PMC12175651
- DOI: 10.1038/s41586-023-05939-3
Lactate regulates cell cycle by remodelling the anaphase promoting complex
Abstract
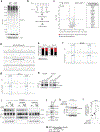
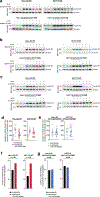
Lactate is abundant in rapidly dividing cells owing to the requirement for elevated glucose catabolism to support proliferation1-6. However, it is not known whether accumulated lactate affects the proliferative state. Here we use a systematic approach to determine lactate-dependent regulation of proteins across the human proteome. From these data, we identify a mechanism of cell cycle regulation whereby accumulated lactate remodels the anaphase promoting complex (APC/C). Remodelling of APC/C in this way is caused by direct inhibition of the SUMO protease SENP1 by lactate. We find that accumulated lactate binds and inhibits SENP1 by forming a complex with zinc in the SENP1 active site. SENP1 inhibition by lactate stabilizes SUMOylation of two residues on APC4, which drives UBE2C binding to APC/C. This direct regulation of APC/C by lactate stimulates timed degradation of cell cycle proteins, and efficient mitotic exit in proliferative human cells. This mechanism is initiated upon mitotic entry when lactate abundance reaches its apex. In this way, accumulation of lactate communicates the consequences of a nutrient-replete growth phase to stimulate timed opening of APC/C, cell division and proliferation. Conversely, persistent accumulation of lactate drives aberrant APC/C remodelling and can overcome anti-mitotic pharmacology via mitotic slippage. In sum, we define a biochemical mechanism through which lactate directly regulates protein function to control the cell cycle and proliferation.
© 2023. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Figures









Comment in
-
Learning the metabolic language of cancer.Nature. 2023 Apr;616(7958):670-671. doi: 10.1038/d41586-023-01024-x. Nature. 2023. PMID: 37072546 No abstract available.
-
Lactate fuels mitosis.Mol Cell. 2023 May 18;83(10):1549-1551. doi: 10.1016/j.molcel.2023.04.013. Mol Cell. 2023. PMID: 37207623 Free PMC article.
References
-
- Brand K, Leibold W, Luppa P, Schoerner C. & Schulz A. Metabolic alterations associated with proliferation of mitogen-activated lymphocytes and of lymphoblastoid cell lines: evaluation of glucose and glutamine metabolism. Immunobiology 173, 23–34, doi: 10.1016/S0171-2985(86)80086-9 (1986). - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

