COVID-19-associated mucormycosis: a systematic review and meta-analysis of 958 cases
- PMID: 36921716
- PMCID: PMC10008766
- DOI: 10.1016/j.cmi.2023.03.008
COVID-19-associated mucormycosis: a systematic review and meta-analysis of 958 cases
Abstract
Background: Mucormycosis, a rare fungal infection, has shown an increase in the number of reported cases during the COVID-19 pandemic.
Objectives: To provide a comprehensive insight into the characteristics of COVID-19-associated mucormycosis, through a systematic review and meta-analysis.
Methods of data synthesis: Demographic information and clinical features were documented for each patient. Logistic regression analysis was used to predict the risk of mortality.
Data sources: PubMed, Scopus, Web of Science, Cochrane, CINAHL, Ovid MEDLINE, and FungiSCOPE.
Study eligibility criteria: Studies reporting individual-level information in patients with adult COVID-19-associated mucormycosis (CAM) between 1 January 2020 and 28 December 2022.
Participants: Adults who developed mucormycosis during or after COVID-19.
Interventions: Patients with and without individual clinical variables were compared.
Assessment of risk of bias: Quality assessment was performed based on the National Institutes of Health quality assessment tool for case series studies.
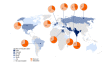
Results: Nine hundred fifty-eight individual cases reported from 45 countries were eligible. 88.1% (844/958) were reported from low- or middle-income countries. Corticosteroid use for COVID-19 (78.5%, 619/789) and diabetes (77.9%, 738/948) were common. Diabetic ketoacidosis (p < 0.001), history of malignancy (p < 0.001), underlying pulmonary (p 0.017), or renal disease (p < 0.001), obesity (p < 0.001), hypertension (p 0.040), age (>65 years) (p 0.001), Aspergillus coinfection (p 0.037), and tocilizumab use during COVID-19 (p 0.018) increased the mortality. CAM occurred on an average of 22 days after COVID-19 and 8 days after hospitalization. Diagnosis of mucormycosis in patients with Aspergillus coinfection and pulmonary mucormycosis was made on average 15.4 days (range, 0-35 days) and 14.0 days (range, 0-53 days) after hospitalization, respectively. Cutaneous mucormycosis accounted for <1% of the cases. The overall mortality rate was 38.9% (303/780).
Conclusion: Mortality of CAM was high, and most reports were from low- or middle-income countries. We detected novel risk factors for CAM, such as older age, specific comorbidities, Aspergillus coinfection, and tocilizumab use, in addition to the previously identified factors.
Keywords: COVID-19; Corticosteroids; Fungal infections; Mucormycosis; Opportunistic infections.
Copyright © 2023 European Society of Clinical Microbiology and Infectious Diseases. Published by Elsevier Ltd. All rights reserved.
Figures



References
-
- World Health Organization . 2020. Who director-general's opening remarks at the media briefing on COVID-19-11 march 2020.https://www.who.int/director-general/speeches/detail/who-director-genera... Available from:
-
- World Health Organization . 2020. Listings of who's a response to covid-19.https://www.who.int/news/item/29-06-2020-covidtimeline Available from:
-
- The centers for Disease Control and Prevention . 2020. Fungal diseases and covid-19.https://www.cdc.gov/fungal/covid-fungal.html Available from:
-
- Prattes J., Wauters J., Giacobbe D.R., Salmanton-García J., Maertens J., Bourgeois M., et al. Risk factors and outcome of pulmonary aspergillosis in critically ill coronavirus disease 2019 patients-a multinational observational study by the European Confederation of Medical Mycology. Clin Microbiol Infect. 2022;28:580–587. doi: 10.1016/j.cmi.2021.08.014. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

