Comparative effectiveness of BNT162b2 versus mRNA-1273 covid-19 vaccine boosting in England: matched cohort study in OpenSAFELY-TPP
- PMID: 36921925
- PMCID: PMC10014664
- DOI: 10.1136/bmj-2022-072808
Comparative effectiveness of BNT162b2 versus mRNA-1273 covid-19 vaccine boosting in England: matched cohort study in OpenSAFELY-TPP
Abstract
Objective: To compare the effectiveness of the BNT162b2 mRNA (Pfizer-BioNTech) and mRNA-1273 (Moderna) covid-19 vaccines during the booster programme in England.
Design: Matched cohort study, emulating a comparative effectiveness trial.
Setting: Linked primary care, hospital, and covid-19 surveillance records available within the OpenSAFELY-TPP research platform, covering a period when the SARS-CoV-2 delta and omicron variants were dominant.
Participants: 3 237 918 adults who received a booster dose of either vaccine between 29 October 2021 and 25 February 2022 as part of the national booster programme in England and who received a primary course of BNT162b2 or ChAdOx1.
Intervention: Vaccination with either BNT162b2 or mRNA-1273 as a booster vaccine dose.
Main outcome measures: Recorded SARS-CoV-2 positive test, covid-19 related hospital admission, covid-19 related death, and non-covid-19 related death at 20 weeks after receipt of the booster dose.
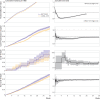
Results: 1 618 959 people were matched in each vaccine group, contributing a total 64 546 391 person weeks of follow-up. The 20 week risks per 1000 for a positive SARS-CoV-2 test were 164.2 (95% confidence interval 163.3 to 165.1) for BNT162b2 and 159.9 (159.0 to 160.8) for mRNA-1273; the hazard ratio comparing mRNA-1273 with BNT162b2 was 0.95 (95% confidence interval 0.95 to 0.96). The 20 week risks per 1000 for hospital admission with covid-19 were 0.75 (0.71 to 0.79) for BNT162b2 and 0.65 (0.61 to 0.69) for mRNA-1273; the hazard ratio was 0.89 (0.82 to 0.95). Covid-19 related deaths were rare: the 20 week risks per 1000 were 0.028 (0.021 to 0.037) for BNT162b2 and 0.024 (0.018 to 0.033) for mRNA-1273; hazard ratio 0.83 (0.58 to 1.19). Comparative effectiveness was generally similar within subgroups defined by the primary course vaccine brand, age, previous SARS-CoV-2 infection, and clinical vulnerability. Relative benefit was similar when vaccines were compared separately in the delta and omicron variant eras.
Conclusions: This matched observational study of adults estimated a modest benefit of booster vaccination with mRNA-1273 compared with BNT162b2 in preventing positive SARS-CoV-2 tests and hospital admission with covid-19 20 weeks after vaccination, during a period of delta followed by omicron variant dominance.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY. No commercial re-use. See rights and permissions. Published by BMJ.
Figures


References
-
- NHS England. NHS begins COVID-19 booster vaccination campaign. 2021. https://www.england.nhs.uk/2021/09/nhs-begins-covid-19-booster-vaccinati....
-
- UK Health Security Agency. COVID-19: the green book, chapter 14a. 2022. https://www.gov.uk/government/publications/covid-19-the-green-book-chapt....
-
- NHS England. NHS booster bookings open to every eligible adult. 2021. https://www.england.nhs.uk/2021/12/nhs-booster-bookings-open-to-every-el....
-
- NHS England. NHS people 40 and over to get their lifesaving booster jab three months on from second dose. 2021. https://www.england.nhs.uk/2021/12/people-40-and-over-to-get-their-lifes....
-
- NHS England. NHS to roll out life-saving booster jab to people aged 30-plus. 2021. https://www.england.nhs.uk/2021/12/nhs-to-roll-out-life-saving-booster-j....
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
