Maternal opioid treatment after delivery and risk of adverse infant outcomes: population based cohort study
- PMID: 36921977
- PMCID: PMC10015218
- DOI: 10.1136/bmj-2022-074005
Maternal opioid treatment after delivery and risk of adverse infant outcomes: population based cohort study
Abstract
Objective: To examine whether maternal opioid treatment after delivery is associated with an increased risk of adverse infant outcomes.
Design: Population based cohort study.
Setting: Ontario, Canada.
Participants: 865 691 mother-infant pairs discharged from hospital alive within seven days of delivery from 1 September 2012 to 31 March 2020. Each mother who filled an opioid prescription within seven days of discharge was propensity score matched to a mother who did not.
Main outcome measures: The primary outcome was hospital readmission of infants for any reason within 30 days of their mother filling an opioid prescription (index date). Infant related secondary outcomes were any emergency department visit, hospital admission for all cause injury, admission to a neonatal intensive care unit, admission with resuscitation or assisted ventilation, and all cause death.
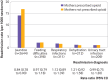
Results: 85 675 mothers (99.8% of the 85 852 mothers prescribed an opioid) who filled an opioid prescription within seven days of discharge after delivery were propensity score matched to 85 675 mothers who did not. Of the infants admitted to hospital within 30 days, 2962 (3.5%) were born to mothers who filled an opioid prescription compared with 3038 (3.5%) born to mothers who did not. Infants of mothers who were prescribed an opioid were no more likely to be admitted to hospital for any reason than infants of mothers who were not prescribed an opioid (hazard ratio 0.98, 95% confidence interval 0.93 to 1.03) and marginally more likely to be taken to an emergency department in the subsequent 30 days (1.04, 1.01 to 1.08), but no differences were found for any other adverse infant outcomes and there were no infant deaths.
Conclusions: Findings from this study suggest no association between maternal opioid prescription after delivery and adverse infant outcomes, including death.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICJME uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: JSZ has received payments for medicolegal opinions regarding the safety and effectiveness of analgesics, including opioids; DNJ is an unpaid member of Physicians for Responsible Opioid Prescribing (PROP). DNJ is also a member of the American College of Medical Toxicology. DNJ has received payment for lectures and medicolegal opinions regarding the safety and effectiveness of analgesics, including opioids. TG is supported by a Canada Research Chair in Drug Policy Research and Evaluation and has received funding from the Ontario Ministry of Health.
Figures

Comment in
-
Opioid analgesia for breastfeeding mothers.BMJ. 2023 Mar 15;380:514. doi: 10.1136/bmj.p514. BMJ. 2023. PMID: 36921958 No abstract available.
References
-
- Centers for Disease Control and Prevention. Breastfeeding Report Card. CDC, 2020. https://www.cdc.gov/breastfeeding/data/reportcard.htm
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
