TMPRSS2 and SARS-CoV-2 SPIKE interaction assay for uHTS
- PMID: 36921991
- PMCID: PMC10399917
- DOI: 10.1093/jmcb/mjad017
TMPRSS2 and SARS-CoV-2 SPIKE interaction assay for uHTS
Abstract
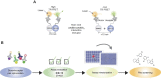
SARS-CoV-2, the coronavirus that causes the disease COVID-19, has claimed millions of lives over the past 2 years. This demands rapid development of effective therapeutic agents that target various phases of the viral replication cycle. The interaction between host transmembrane serine protease 2 (TMPRSS2) and viral SPIKE protein is an important initial step in SARS-CoV-2 infection, offering an opportunity for therapeutic development of viral entry inhibitors. Here, we report the development of a time-resolved fluorescence/Förster resonance energy transfer (TR-FRET) assay for monitoring the TMPRSS2-SPIKE interaction in lysate from cells co-expressing these proteins. The assay was configured in a 384-well-plate format for high-throughput screening with robust assay performance. To enable large-scale compound screening, we further miniaturized the assay into 1536-well ultrahigh-throughput screening (uHTS) format. A pilot screen demonstrated the utilization of the assay for uHTS. Our optimized TR-FRET uHTS assay provides an enabling platform for expanded screening campaigns to discover new classes of small-molecule inhibitors that target the SPIKE and TMPRSS2 protein-protein interaction.
Keywords: COVID-19; SARS-CoV-2; SPIKE; TMPRSS2; TR-FRET; high-throughput screening; protein–protein interaction.
© The Author(s) (2023). Published by Oxford University Press on behalf of Journal of Molecular Cell Biology, CEMCS, CAS.
Figures



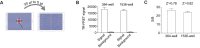



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

