ALOX5-mediated ferroptosis acts as a distinct cell death pathway upon oxidative stress in Huntington's disease
- PMID: 36921996
- PMCID: PMC10111862
- DOI: 10.1101/gad.350211.122
ALOX5-mediated ferroptosis acts as a distinct cell death pathway upon oxidative stress in Huntington's disease
Abstract
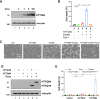
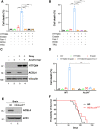
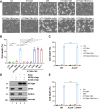
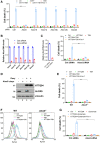
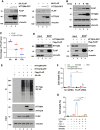
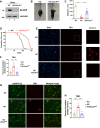
Although it is well established that Huntington's disease (HD) is mainly caused by polyglutamine-expanded mutant huntingtin (mHTT), the molecular mechanism of mHTT-mediated actions is not fully understood. Here, we showed that expression of the N-terminal fragment containing the expanded polyglutamine (HTTQ94) of mHTT is able to promote both the ACSL4-dependent and the ACSL4-independent ferroptosis. Surprisingly, inactivation of the ACSL4-dependent ferroptosis fails to show any effect on the life span of Huntington's disease mice. Moreover, by using RNAi-mediated screening, we identified ALOX5 as a major factor required for the ACSL4-independent ferroptosis induced by HTTQ94. Although ALOX5 is not required for the ferroptotic responses triggered by common ferroptosis inducers such as erastin, loss of ALOX5 expression abolishes HTTQ94-mediated ferroptosis upon reactive oxygen species (ROS)-induced stress. Interestingly, ALOX5 is also required for HTTQ94-mediated ferroptosis in neuronal cells upon high levels of glutamate. Mechanistically, HTTQ94 activates ALOX5-mediated ferroptosis by stabilizing FLAP, an essential cofactor of ALOX5-mediated lipoxygenase activity. Notably, inactivation of the Alox5 gene abrogates the ferroptosis activity in the striatal neurons from the HD mice; more importantly, loss of ALOX5 significantly ameliorates the pathological phenotypes and extends the life spans of these HD mice. Taken together, these results demonstrate that ALOX5 is critical for mHTT-mediated ferroptosis and suggest that ALOX5 is a potential new target for Huntington's disease.
Keywords: ACSL4; ALOX5; GPX4; HTT; ROS; ferroptosis; oxidative stress.
© 2023 Song et al.; Published by Cold Spring Harbor Laboratory Press.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
