Monocyte migration profiles define disease severity in acute COVID-19 and unique features of long COVID
- PMID: 36922030
- PMCID: PMC10040898
- DOI: 10.1183/13993003.02226-2022
Monocyte migration profiles define disease severity in acute COVID-19 and unique features of long COVID
Abstract
Background: COVID-19 is associated with a dysregulated immune response but it is unclear how immune dysfunction contributes to the chronic morbidity persisting in many COVID-19 patients during convalescence (long COVID).
Methods: We assessed phenotypical and functional changes of monocytes in COVID-19 patients during hospitalisation and up to 9 months of convalescence following COVID-19, respiratory syncytial virus or influenza A. Patients with progressive fibrosing interstitial lung disease were included as a positive control for severe, ongoing lung injury.
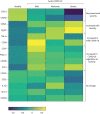
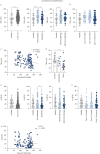
Results: Monocyte alterations in acute COVID-19 patients included aberrant expression of leukocyte migration molecules, continuing into convalescence (n=142) and corresponding with specific symptoms of long COVID. Long COVID patients with unresolved lung injury, indicated by sustained shortness of breath and abnormal chest radiology, were defined by high monocyte expression of C-X-C motif chemokine receptor 6 (CXCR6) (p<0.0001) and adhesion molecule P-selectin glycoprotein ligand 1 (p<0.01), alongside preferential migration of monocytes towards the CXCR6 ligand C-X-C motif chemokine ligand 16 (CXCL16) (p<0.05), which is abundantly expressed in the lung. Monocyte CXCR6 and lung CXCL16 were heightened in patients with progressive fibrosing interstitial lung disease (p<0.001), confirming a role for the CXCR6-CXCL16 axis in ongoing lung injury. Conversely, monocytes from long COVID patients with ongoing fatigue exhibited a sustained reduction of the prostaglandin-generating enzyme cyclooxygenase 2 (p<0.01) and CXCR2 expression (p<0.05). These monocyte changes were not present in respiratory syncytial virus or influenza A convalescence.
Conclusions: Our data define unique monocyte signatures that define subgroups of long COVID patients, indicating a key role for monocyte migration in COVID-19 pathophysiology. Targeting these pathways may provide novel therapeutic opportunities in COVID-19 patients with persistent morbidity.
Copyright ©The authors 2023.
Conflict of interest statement
Conflict of interest: G. Lindergard is co-founder and scientific advisory board member of Gritstone Bio Inc., which is a public company that develops therapeutic vaccines for the treatment of cancer and infectious diseases, including COVID-19. The other authors declare that they have no competing interests.
Figures






Comment in
-
Long COVID: clues about causes.Eur Respir J. 2023 May 11;61(5):2300409. doi: 10.1183/13993003.00409-2023. Print 2023 May. Eur Respir J. 2023. PMID: 36958743 Free PMC article.
References
-
- World Health Organization . World Health Organization (COVID-19) Dashboard. https://covid19.who.int/ Date last accessed: 13 April 2023.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
