Endemic mycoses in children in North America: a review of radiologic findings
- PMID: 36922418
- PMCID: PMC10017348
- DOI: 10.1007/s00247-023-05636-3
Endemic mycoses in children in North America: a review of radiologic findings
Abstract
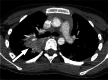
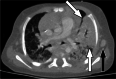
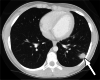
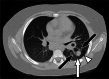
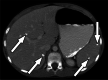
Clinically significant endemic mycoses (fungal infections) in the United States (U.S.) include Blastomyces dermatitidis, Histoplasma capsulatum, and Coccidioides immitis/posadasii. While the majority of infections go clinically unnoticed, symptomatic disease can occur in immunocompromised or hospitalized patients, and occasionally in immune-competent individuals. Clinical manifestations vary widely and their diagnosis may require fungal culture, making the rapid diagnosis a challenge. Imaging can be helpful in making a clinical diagnosis prior to laboratory confirmation, as well as assist in characterizing disease extent and severity. In this review, we discuss the three major endemic fungal infections that occur in the U.S., including mycology, epidemiology, clinical presentations, and typical imaging features with an emphasis on the pediatric population.
Keywords: Blastomycosis; Children; Coccidioidomycosis; Endemic fungal infection; Histoplasmosis; Imaging.
© 2023. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
None
Figures














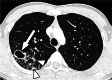
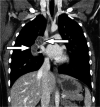


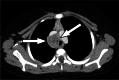



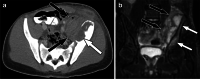











References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

