Selenium Nanoparticles Modulate Steroidogenesis-Related Genes and Improve Ovarian Functions via Regulating Androgen Receptors Expression in Polycystic Ovary Syndrome Rat Model
- PMID: 36922476
- PMCID: PMC10620277
- DOI: 10.1007/s12011-023-03616-0
Selenium Nanoparticles Modulate Steroidogenesis-Related Genes and Improve Ovarian Functions via Regulating Androgen Receptors Expression in Polycystic Ovary Syndrome Rat Model
Abstract
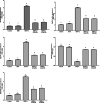
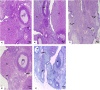
Polycystic ovary syndrome (PCOS) occurs during the reproductive period in women and is characterized by reproductive, endocrine, and metabolic disorders. Androgen plays a decisive role in its pathogenesis due to the interaction between hyperandrogenism and insulin resistance, which might be improved by selenium nanoparticles (SeNPs). The present study aimed to clarify the effect of SeNPs on androgen synthesis and action in the PCOS model and the resulting effect on ovarian function. Fifty-five 7-week-old female albino rats (90-105 g) were divided equally into five groups: control (C), fed a standard diet for 11 weeks; high-fat diet (HFD) group, fed HFD for 11 weeks; HFD and letrozole (L) (HFD + L), fed HFD for 11 weeks and administrated orally with L, at a daily dose of 1 mg/kg BW, for three weeks from the 7th to 9th week of the trial; HFD + L + 0.1SeNPs and HFD + L + 0.2SeNPs groups, treated the same as HFD + L group and orally gavaged SeNPs at daily doses of 0.1 and 0.2 mg/kg BW, respectively, during the last 14 day of the experiment. Daily determination of estrous cycle was performed, and at the end of the experimental period, BMI, serum glucose, insulin, HOMA-IR, lipid profile, sex hormones, TNF-α, IL6, oxidative stress biomarkers, ovarian mRNA expression of different proteins and enzymes involved in steroidogenesis, pathological examination, and immunohistochemical staining for androgen receptor (AR) were evaluated. Treatment of SeNPs restored estrous cyclicity, decreased BMI, and insulin resistance, improved dyslipidemia, reduced serum testosterone, and improved ovarian histopathology in PCOS rats. Furthermore, the anti-inflammatory and antioxidant impacts of SeNPs were remarkably noticed. Administration of SeNPs decreased androgen synthesis and expression of ovarian AR protein by decreasing the mRNA expression of STAR, Cyp11A1, Cyp17A1, and HSD17B3 and increasing the expression of Cyp19α1. Conclusively, SeNPs decreased androgen synthesis and blocked the vicious circle initiated by excessive androgen secretion via decreased AR expression. Thus, it may effectively treat PCOS cases by eliminating its reproductive, endocrine, and metabolic dysfunctions.
Keywords: Androgen receptors; Ovarian histopathology and immunohistochemistry; PCOS; Selenium nanoparticles; Steroidogenesis; mRNA expression.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- de Guevara AL, Fux-Otta C, Crisosto N, de Mereshian PS, Echiburú B, Iraci G, Perez-Bravo F, Sir-Petermann T. Metabolic profile of the different phenotypes of polycystic ovary syndrome in two Latin American populations. Fertil Steril. 2014;101(6):1732–1739. e2. doi: 10.1016/j.fertnstert.2014.02.020. - DOI - PubMed
-
- Lazaros L, Xita N, Hatzi E, Takenaka A, Kaponis A, Makrydimas G, Sofikitis N, Stefos T, Zikopoulos K, Georgiou I. CYP19 gene variants affect the assisted reproduction outcome of women with polycystic ovary syndrome. Gynecol Endocrinol. 2013;29(5):478–482. doi: 10.3109/09513590.2013.774359. - DOI - PubMed
-
- Caldwell A, Middleton L, Jimenez M, Desai R, McMahon A, Allan C, Handelsman D, Walters K. Characterization of reproductive, metabolic, and endocrine features of polycystic ovary syndrome in female hyperandrogenic mouse models. Endocrinology. 2014;155(8):3146–3159. doi: 10.1210/en.2014-1196. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

