Engineered exosomes from different sources for cancer-targeted therapy
- PMID: 36922504
- PMCID: PMC10017761
- DOI: 10.1038/s41392-023-01382-y
Engineered exosomes from different sources for cancer-targeted therapy
Abstract
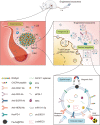
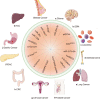
Exosome is a subgroup of extracellular vesicles, which has been serving as an efficient therapeutic tool for various diseases. Engineered exosomes are the sort of exosomes modified with surface decoration and internal therapeutic molecules. After appropriate modification, engineered exosomes are able to deliver antitumor drugs to tumor sites efficiently and precisely with fewer treatment-related adverse effects. However, there still exist many challenges for the clinical translation of engineered exosomes. For instance, what sources and modification strategies could endow exosomes with the most efficient antitumor activity is still poorly understood. Additionally, how to choose appropriately engineered exosomes in different antitumor therapies is another unresolved problem. In this review, we summarized the characteristics of engineered exosomes, especially the spatial and temporal properties. Additionally, we concluded the recent advances in engineered exosomes in the cancer fields, including the sources, isolation technologies, modification strategies, and labeling and imaging methods of engineered exosomes. Furthermore, the applications of engineered exosomes in different antitumor therapies were summarized, such as photodynamic therapy, gene therapy, and immunotherapy. Consequently, the above provides the cancer researchers in this community with the latest ideas on engineered exosome modification and new direction of new drug development, which is prospective to accelerate the clinical translation of engineered exosomes for cancer-targeted therapy.
© 2023. The Author(s).
Conflict of interest statement
The authors have no financial conflict of interest.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

