Blocking NS3-NS4B interaction inhibits dengue virus in non-human primates
- PMID: 36922586
- PMCID: PMC10033419
- DOI: 10.1038/s41586-023-05790-6
Blocking NS3-NS4B interaction inhibits dengue virus in non-human primates
Abstract
Dengue is a major health threat and the number of symptomatic infections caused by the four dengue serotypes is estimated to be 96 million1 with annually around 10,000 deaths2. However, no antiviral drugs are available for the treatment or prophylaxis of dengue. We recently described the interaction between non-structural proteins NS3 and NS4B as a promising target for the development of pan-serotype dengue virus (DENV) inhibitors3. Here we present JNJ-1802-a highly potent DENV inhibitor that blocks the NS3-NS4B interaction within the viral replication complex. JNJ-1802 exerts picomolar to low nanomolar in vitro antiviral activity, a high barrier to resistance and potent in vivo efficacy in mice against infection with any of the four DENV serotypes. Finally, we demonstrate that the small-molecule inhibitor JNJ-1802 is highly effective against viral infection with DENV-1 or DENV-2 in non-human primates. JNJ-1802 has successfully completed a phase I first-in-human clinical study in healthy volunteers and was found to be safe and well tolerated4. These findings support the further clinical development of JNJ-1802, a first-in-class antiviral agent against dengue, which is now progressing in clinical studies for the prevention and treatment of dengue.
© 2023. The Author(s).
Conflict of interest statement
B.K., J.-F.B., T.H.M.J., P.R., D.B. and A.M. have been named inventors in a patent application claiming the discovery of this class of antiviral molecules as DENV replication inhibitors (WO 2016/180696), which was filed by Applicants Janssen Pharmaceuticals, Inc. and Katholieke Universiteit Leuven, and has been granted in certain countries. O.G., B.K., B.S., J.-F.B., T.H.M.J., M.V.L., S.J.F.K. and J.N. have been named inventors in a pending patent application relating to the use of substituted indole derivatives and substituted indoline derivatives in the manufacture of a medicament for the treatment or the prevention of dengue disease in an individual at risk of being infected by DENV and to a method for the treatment or the prevention of dengue in an individual at risk of being infected by DENV, which was filed by Applicants Janssen Pharmaceuticals, Inc. and Katholieke Universiteit Leuven (WO 2021/094563). O.G., B.K., L.V.W., O.A., R.S., S.L.-D., M.C., K.T., B.S., O.L., L.T., S.D.M., T.H.M.J., K.S. and M.V.L. are all full-time employees of Janssen and potential stockholders of Johnson and Johnson. The other authors declare no competing interests.
Figures
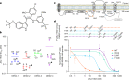


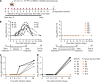





Comment in
-
DENV inhibitor effective in non-human primates.Nat Rev Drug Discov. 2023 May;22(5):355. doi: 10.1038/d41573-023-00053-5. Nat Rev Drug Discov. 2023. PMID: 37020012 No abstract available.
References
-
- Ackaert, O. et al. Safety, tolerability and pharmacokinetics of a novel pan-serotype dengue antiviral small molecule in a phase 1, double-blind, randomized, dose-escalation study [abstract 0582]. in The American Society of Tropical Medicine & Hygiene (ASTMH) Virtual Meeting Abstract Book (2021).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

