Fast and sensitive GCaMP calcium indicators for imaging neural populations
- PMID: 36922596
- PMCID: PMC10060165
- DOI: 10.1038/s41586-023-05828-9
Fast and sensitive GCaMP calcium indicators for imaging neural populations
Abstract
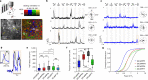
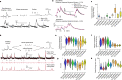
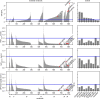
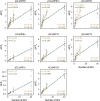
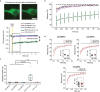
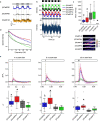
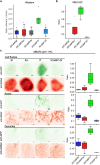
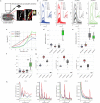
Calcium imaging with protein-based indicators1,2 is widely used to follow neural activity in intact nervous systems, but current protein sensors report neural activity at timescales much slower than electrical signalling and are limited by trade-offs between sensitivity and kinetics. Here we used large-scale screening and structure-guided mutagenesis to develop and optimize several fast and sensitive GCaMP-type indicators3-8. The resulting 'jGCaMP8' sensors, based on the calcium-binding protein calmodulin and a fragment of endothelial nitric oxide synthase, have ultra-fast kinetics (half-rise times of 2 ms) and the highest sensitivity for neural activity reported for a protein-based calcium sensor. jGCaMP8 sensors will allow tracking of large populations of neurons on timescales relevant to neural computation.
© 2023. The Author(s).
Conflict of interest statement
Y.Z., E.R.S., J.P.H., I.K., K.S. and L.L.L. are inventors of US Patent Application 63082222, ‘Genetically Encoded Calcium Indicators and Methods of Use’, which covers the jGCaMP8 sensors and is assigned to HHMI. The remaining authors declare no competing interests.
Figures








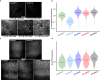
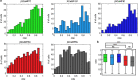

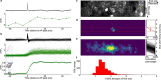
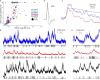



Comment in
-
Fastest-ever calcium sensors broaden the potential of neuronal imaging.Nature. 2023 Mar;615(7954):804-805. doi: 10.1038/d41586-023-00704-y. Nature. 2023. PMID: 36922656 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

