The dietary sweetener sucralose is a negative modulator of T cell-mediated responses
- PMID: 36922598
- PMCID: PMC10033444
- DOI: 10.1038/s41586-023-05801-6
The dietary sweetener sucralose is a negative modulator of T cell-mediated responses
Abstract
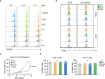
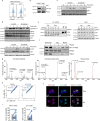
Artificial sweeteners are used as calorie-free sugar substitutes in many food products and their consumption has increased substantially over the past years1. Although generally regarded as safe, some concerns have been raised about the long-term safety of the consumption of certain sweeteners2-5. In this study, we show that the intake of high doses of sucralose in mice results in immunomodulatory effects by limiting T cell proliferation and T cell differentiation. Mechanistically, sucralose affects the membrane order of T cells, accompanied by a reduced efficiency of T cell receptor signalling and intracellular calcium mobilization. Mice given sucralose show decreased CD8+ T cell antigen-specific responses in subcutaneous cancer models and bacterial infection models, and reduced T cell function in models of T cell-mediated autoimmunity. Overall, these findings suggest that a high intake of sucralose can dampen T cell-mediated responses, an effect that could be used in therapy to mitigate T cell-dependent autoimmune disorders.
© 2023. The Author(s).
Conflict of interest statement
K.H.V. is on the board of directors and shareholder of Bristol Myers Squibb and on the scientific advisory board (with stock options) of PMV Pharma, RAZE Therapeutics, Volastra Pharmaceuticals and Kovina Therapeutics. She is on the scientific advisory board of Ludwig Cancer and a co-founder and consultant of Faeth Therapeutics. She has been in receipt of research funding from Astex Pharmaceuticals and AstraZeneca and contributed to CRUK Cancer Research Technology filing of patent application WO/2017/144877. J.B. is funded by a fellowship from the Kuok Family, who did not influence any aspects of the research. The other authors declare no competing interests.
Figures











Comment in
-
Sucralose: not sweet enough for T cells.Nat Rev Immunol. 2023 May;23(5):270. doi: 10.1038/s41577-023-00873-x. Nat Rev Immunol. 2023. PMID: 37012434 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

