Exogenous mitochondrial transplantation improves survival and neurological outcomes after resuscitation from cardiac arrest
- PMID: 36922820
- PMCID: PMC10018842
- DOI: 10.1186/s12916-023-02759-0
Exogenous mitochondrial transplantation improves survival and neurological outcomes after resuscitation from cardiac arrest
Abstract
Background: Mitochondrial transplantation (MTx) is an emerging but poorly understood technology with the potential to mitigate severe ischemia-reperfusion injuries after cardiac arrest (CA). To address critical gaps in the current knowledge, we test the hypothesis that MTx can improve outcomes after CA resuscitation.
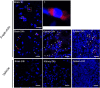
Methods: This study consists of both in vitro and in vivo studies. We initially examined the migration of exogenous mitochondria into primary neural cell culture in vitro. Exogenous mitochondria extracted from the brain and muscle tissues of donor rats and endogenous mitochondria in the neural cells were separately labeled before co-culture. After a period of 24 h following co-culture, mitochondrial transfer was observed using microscopy. In vitro adenosine triphosphate (ATP) contents were assessed between freshly isolated and frozen-thawed mitochondria to compare their effects on survival. Our main study was an in vivo rat model of CA in which rats were subjected to 10 min of asphyxial CA followed by resuscitation. At the time of achieving successful resuscitation, rats were randomly assigned into one of three groups of intravenous injections: vehicle, frozen-thawed, or fresh viable mitochondria. During 72 h post-CA, the therapeutic efficacy of MTx was assessed by comparison of survival rates. The persistence of labeled donor mitochondria within critical organs of recipient animals 24 h post-CA was visualized via microscopy.
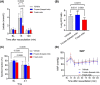
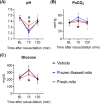
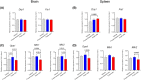
Results: The donated mitochondria were successfully taken up into cultured neural cells. Transferred exogenous mitochondria co-localized with endogenous mitochondria inside neural cells. ATP content in fresh mitochondria was approximately four times higher than in frozen-thawed mitochondria. In the in vivo survival study, freshly isolated functional mitochondria, but not frozen-thawed mitochondria, significantly increased 72-h survival from 55 to 91% (P = 0.048 vs. vehicle). The beneficial effects on survival were associated with improvements in rapid recovery of arterial lactate and glucose levels, cerebral microcirculation, lung edema, and neurological function. Labeled mitochondria were observed inside the vital organs of the surviving rats 24 h post-CA.
Conclusions: MTx performed immediately after resuscitation improved survival and neurological recovery in post-CA rats. These results provide a foundation for future studies to promote the development of MTx as a novel therapeutic strategy to save lives currently lost after CA.
Keywords: Cardiac arrest; Ischemia and reperfusion; Mitochondria; Mitochondrial transplantation.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Neumar RW, Nolan JP, Adrie C, Aibiki M, Berg RA, Bottiger BW, Callaway C, Clark RS, Geocadin RG, Jauch EC, et al. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A consensus statement from the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation. 2008;118(23):2452–2483. doi: 10.1161/CIRCULATIONAHA.108.190652. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

