Revisiting how we perform late gadolinium enhancement CMR: insights gleaned over 25 years of clinical practice
- PMID: 36922844
- PMCID: PMC10018965
- DOI: 10.1186/s12968-023-00925-0
Revisiting how we perform late gadolinium enhancement CMR: insights gleaned over 25 years of clinical practice
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



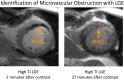
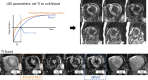
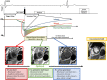
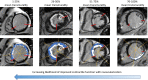
Similar articles
-
Relationship of Myocardial Gadolinium Enhancement to Late Clinical Outcomes: Implications for the COVID-19 era.Heart Lung Circ. 2022 Mar;31(3):e29-e30. doi: 10.1016/j.hlc.2021.08.010. Epub 2021 Nov 1. Heart Lung Circ. 2022. PMID: 34736825 Free PMC article. No abstract available.
-
Myocardial late gadolinium enhancement in specific cardiomyopathies by cardiovascular magnetic resonance: a preliminary experience.J Cardiovasc Med (Hagerstown). 2007 Dec;8(12):1076-9. doi: 10.2459/01.JCM.0000296538.82763.f0. J Cardiovasc Med (Hagerstown). 2007. PMID: 18163027
-
Late Gadolinium Enhancement Cardiac Magnetic Resonance Tissue Characterization for Cancer-Associated Cardiac Masses: Metabolic and Prognostic Manifestations in Relation to Whole-Body Positron Emission Tomography.J Am Heart Assoc. 2019 May 21;8(10):e011709. doi: 10.1161/JAHA.118.011709. J Am Heart Assoc. 2019. PMID: 31072171 Free PMC article.
-
Prognostic Value of Late Gadolinium Enhancement Detected on Cardiac Magnetic Resonance in Cardiac Sarcoidosis.JACC Cardiovasc Imaging. 2023 Mar;16(3):345-357. doi: 10.1016/j.jcmg.2022.10.018. Epub 2023 Jan 11. JACC Cardiovasc Imaging. 2023. PMID: 36752432
-
Machine Learning-Based Segmentation of Left Ventricular Myocardial Fibrosis from Magnetic Resonance Imaging.Curr Cardiol Rep. 2020 Jun 19;22(8):65. doi: 10.1007/s11886-020-01321-1. Curr Cardiol Rep. 2020. PMID: 32562100 Review.
Cited by
-
Cardiac Magnetic Resonance for Structural Aortic Valve Stenosis Procedures.J Clin Med. 2024 Sep 1;13(17):5184. doi: 10.3390/jcm13175184. J Clin Med. 2024. PMID: 39274397 Free PMC article. Review.
-
H-ferritin-nanocaged gadolinium nanoparticles for ultra-sensitive MR molecular imaging.Theranostics. 2024 Feb 24;14(5):1956-1965. doi: 10.7150/thno.93856. eCollection 2024. Theranostics. 2024. PMID: 38505606 Free PMC article.
-
Quantification of Replacement Fibrosis in Aortic Stenosis: A Narrative Review on the Utility of Cardiovascular Magnetic Resonance Imaging.Diagnostics (Basel). 2024 Oct 31;14(21):2435. doi: 10.3390/diagnostics14212435. Diagnostics (Basel). 2024. PMID: 39518402 Free PMC article. Review.
-
Myocardial Damage Patterns in Patients with Left Ventricular Systolic Dysfunction with and Without Coronary Artery Disease Referred for Cardiac Magnetic Resonance.Biomedicines. 2025 Jul 1;13(7):1612. doi: 10.3390/biomedicines13071612. Biomedicines. 2025. PMID: 40722685 Free PMC article.
-
Pre-hospital pulse glucocorticoid therapy in patients with ST-segment elevation myocardial infarction transferred for primary percutaneous coronary intervention: a randomized controlled trial (PULSE-MI).Trials. 2023 Dec 15;24(1):808. doi: 10.1186/s13063-023-07830-y. Trials. 2023. PMID: 38102687 Free PMC article. Clinical Trial.
References
-
- Kim RJ, Albert TS, Wible JH, Elliott MD, Allen JC, Lee JC, Parker M, Napoli A, Judd RM. Gadoversetamide Myocardial Infarction Imaging I. Performance of delayed-enhancement magnetic resonance imaging with gadoversetamide contrast for the detection and assessment of myocardial infarction: an international, multicenter, double-blinded, randomized trial. Circulation. 2008;117:629–637. doi: 10.1161/CIRCULATIONAHA.107.723262. - DOI - PubMed
-
- Weinreb JC, Rodby RA, Yee J, Wang CL, Fine D, McDonald RJ, Perazella MA, Dillman JR, Davenport MS. Use of intravenous gadolinium-based contrast media in patients with kidney disease: Consensus Statements from the American College of Radiology and the National Kidney Foundation. Radiology. 2021;298:28–35. doi: 10.1148/radiol.2020202903. - DOI - PubMed
-
- Sievers B, Elliott MD, Hurwitz LM, Albert TS, Klem I, Rehwald WG, Parker MA, Judd RM, Kim RJ. Rapid detection of myocardial infarction by subsecond, free-breathing delayed contrast-enhancement cardiovascular magnetic resonance. Circulation. 2007;115:236–244. doi: 10.1161/CIRCULATIONAHA.106.635409. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

