Evaluating interventions to reduce behaviour associated with HCV reinfection in men who have sex with men: study protocol for a non-blinded, phase 2, randomised trial
- PMID: 36922871
- PMCID: PMC10015546
- DOI: 10.1186/s13063-023-07161-y
Evaluating interventions to reduce behaviour associated with HCV reinfection in men who have sex with men: study protocol for a non-blinded, phase 2, randomised trial
Abstract
Background: As highly effective therapy against hepatitis C virus (HCV) infection is available with rapid uptake, there is newfound optimism for HCV elimination. Nevertheless, certain key populations have a high risk of HCV reinfection, in particular men who have sex with men (MSM) in Western European countries. Modelling data indicate that HCV elimination will not be feasible without reduction in risk behaviour, thus supporting the need for effective interventions aimed at reducing risk behaviour and preventing reinfections in MSM.
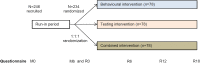
Methods: The ICECREAM study is an international, multi-centred, phase 2, 3-arm randomised trial comparing run-in and intervention periods enrolling MSM with a history of a cured or spontaneously cleared HCV infection. Individuals are followed in routine care for 6 months (i.e. run-in period) and then randomly allocated (1:1:1) to one of the following: a tailored, interactive online risk-reduction behavioural intervention, a validated home-based HCV-RNA self-sampling test service using dried blood spots, or a combination of both. After randomisation, individuals are followed every 6 months until 18 months (i.e. intervention period). Interventions are delivered in addition to standard of care. Online questionnaire measuring risk behaviour over the past 6 months is administered at every visit. The primary outcome is the proportion at risk of HCV infection during run-in versus intervention periods assessed by using the HCV-MOSAIC risk score. The risk score consists of six self-reported HCV-related risk behaviours. Secondary outcomes include incidence of HCV reinfection, changes in the individual risk behaviour items and changes in sexual well-being since changes in sexual behaviour may have an impact on sexual experience. Two hundred forty-six MSM aged 18 years or older will be invited to participate.
Discussion: The ICECREAM study is a trial aimed at establishing interventions that could effectively decrease the incidence of HCV re-infection in MSM with a previous HCV infection. By offering an online behavioural risk-reduction intervention and HCV-RNA self-sampling, both of which are aimed to influence risk behaviour, we are able to provide products to at-risk MSM that could further reduce population-level HCV incidence and ultimately help reach HCV micro-elimination.
Trial registration: ClinicalTrials.gov NCT04156945. Registered on November 8, 2019.
Keywords: HCV; Intervention; Randomised trial; Reinfection; Risk behaviour.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

