Astrocytic APOE4 removal confers cerebrovascular protection despite increased cerebral amyloid angiopathy
- PMID: 36922879
- PMCID: PMC10018855
- DOI: 10.1186/s13024-023-00610-x
Astrocytic APOE4 removal confers cerebrovascular protection despite increased cerebral amyloid angiopathy
Abstract
Background: Alzheimer Disease (AD) and cerebral amyloid angiopathy (CAA) are both characterized by amyloid-β (Aβ) accumulation in the brain, although Aβ deposits mostly in the brain parenchyma in AD and in the cerebrovasculature in CAA. The presence of CAA can exacerbate clinical outcomes of AD patients by promoting spontaneous intracerebral hemorrhage and ischemia leading to CAA-associated cognitive decline. Genetically, AD and CAA share the ε4 allele of the apolipoprotein E (APOE) gene as the strongest genetic risk factor. Although tremendous efforts have focused on uncovering the role of APOE4 on parenchymal plaque pathogenesis in AD, mechanistic studies investigating the role of APOE4 on CAA are still lacking. Here, we addressed whether abolishing APOE4 generated by astrocytes, the major producers of APOE, is sufficient to ameliorate CAA and CAA-associated vessel damage.
Methods: We generated transgenic mice that deposited both CAA and plaques in which APOE4 expression can be selectively suppressed in astrocytes. At 2-months-of-age, a timepoint preceding CAA and plaque formation, APOE4 was removed from astrocytes of 5XFAD APOE4 knock-in mice. Mice were assessed at 10-months-of-age for Aβ plaque and CAA pathology, gliosis, and vascular integrity.
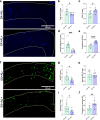
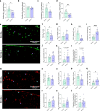
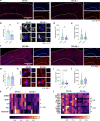
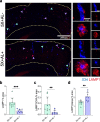
Results: Reducing the levels of APOE4 in astrocytes shifted the deposition of fibrillar Aβ from the brain parenchyma to the cerebrovasculature. However, despite increased CAA, astrocytic APOE4 removal reduced overall Aβ-mediated gliosis and also led to increased cerebrovascular integrity and function in vessels containing CAA.
Conclusion: In a mouse model of CAA, the reduction of APOE4 derived specifically from astrocytes, despite increased fibrillar Aβ deposition in the vasculature, is sufficient to reduce Aβ-mediated gliosis and cerebrovascular dysfunction.
Keywords: APOE; Amyloid-β; Astrocyte; Cerebral amyloid angiopathy; Cerebrovasculature.
© 2023. The Author(s).
Conflict of interest statement
M.X. is an employee of Genentech. D.M.H. is as an inventor on a patent licensed by Washington University to NextCure on the therapeutic use of anti-APOE antibodies. D.M.H. co-founded and is on the scientific advisory board of C2N Diagnostics. D.M.H. is on the scientific advisory board of Denali, Genentech, and Cajal Neuroscience and consults for Alector. All other authors declare that they have no competing interests to disclose.
Figures

References
-
- Hanseeuw BJ, Betensky RA, Jacobs HIL, Schultz AP, Sepulcre J, Becker JA, Cosio DMO, Farrell M, Quiroz YT, Mormino EC, Buckley RF, Papp KV, Amariglio RA, Dewachter I, Ivanoiu A, Huijbers W, Hedden T, Marshall GA, Chhatwal JP, Rentz DM, Sperling RA, Johnson K. Association of Amyloid and Tau with cognition in preclinical alzheimer disease: a longitudinal study. JAMA Neurol. 2019;76:915–924. doi: 10.1001/jamaneurol.2019.1424. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

