Population pharmacokinetics and exposure-response analysis of a single dose of sotrovimab in the early treatment of patients with mild to moderate COVID-19
- PMID: 36922886
- PMCID: PMC10272298
- DOI: 10.1002/psp4.12958
Population pharmacokinetics and exposure-response analysis of a single dose of sotrovimab in the early treatment of patients with mild to moderate COVID-19
Abstract
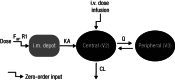
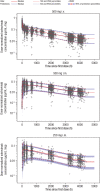
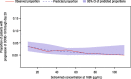
Sotrovimab is a recombinant human monoclonal antibody that has been shown to prevent progression to hospitalization or death in non-hospitalized high-risk patients with mild to moderate coronavirus disease 2019 following either intravenous (i.v.) or intramuscular (i.m.) administration. Population pharmacokinetic (PopPK) and exposure-response (ER) analyses were performed to characterize single dose sotrovimab pharmacokinetics (PK) and the relationship between exposure and response (probability of progression), as well as covariates that may contribute to between-participant variability in sotrovimab PK and efficacy following i.v. or i.m. administration. Sotrovimab PK was described by a two-compartment model with linear elimination; i.m. absorption was characterized by a sigmoid absorption model. PopPK covariate analysis led to the addition of the effect of body weight on systemic clearance and peripheral volume of distribution, sex on i.m. bioavailability and first-order absorption rate (KA), and body mass index on KA. However, the magnitude of covariate effect was not pronounced and was therefore not expected to be clinically relevant based on available data to date. For ER analysis, sotrovimab exposure measures were predicted using the final PopPK model. An ER model was developed using the exposure measure of sotrovimab concentration at 168 h that described the relationship between exposure and probability of progression within the ER dataset for COMET-TAIL. The number of risk factors (≤1 vs. >1) was incorporated as an additive shift on the model-estimated placebo response but had no impact on overall drug response. Limitations in the ER model may prevent generalization of these results to describe the sotrovimab exposure-progression relationship across severe acute respiratory syndrome-coronavirus 2 variants.
© 2023 Vir Biotechnology. CPT: Pharmacometrics & Systems Pharmacology published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
Jennifer E. Sager, Asma El‐Zailik, Melissa Aldinger, Elizabeth L. Alexander, Wendy W. Yeh, Erik Mogalian, Chad Garner, and Maribel Reyes are employees of Vir Biotechnology and report stock ownership in Vir Biotechnology; third‐party funding from GSK to Vir Biotechnology for the submitted work; and patent applications planned, pending, or issued on or related to the content of this manuscript. Julie Passarell and Stefan Roepcke are employees of Cognigen Division, Simulations Plus, Inc. and report third‐party funding from GSK to Vir Biotechnology for the submitted work; consulting fees were paid to Cognigen by Vir Biotechnology, Inc. for the submitted work. Xiaobin Li, Ahmed Nader, Andrew Skingsley, and Amanda Peppercorn are employees of GSK and report stock ownership in GSK and third‐party funding from GSK to Vir Biotechnology for the submitted work. Adrienne E. Shapiro reports acting as a trial investigator for Vir Biotechnology and receiving nonfinancial support from Vir Biotechnology during the conduct of the study.
Figures

References
-
- Nguyen JL, Alfred T, Reimbaeva M, et al. Population attributable fractions of underlying medical conditions for coronavirus disease 2019 (COVID‐19) diagnosis and COVID‐19 hospitalizations, ventilations, and deaths among adults in the United States. Open Forum Infect Dis. 2022;9(5):ofac099. - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical

