Advances in the synthesis of nitrogen-containing heterocyclic compounds by in situ benzyne cycloaddition
- PMID: 36922948
- PMCID: PMC10010163
- DOI: 10.1039/d3ra00400g
Advances in the synthesis of nitrogen-containing heterocyclic compounds by in situ benzyne cycloaddition
Abstract
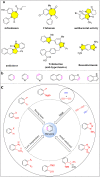
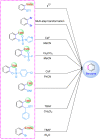
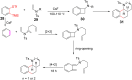
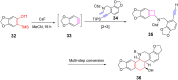
Nitrogen-containing heterocyclic compounds are prevalent in various natural products, medicines, agrochemicals, and organic functional materials. Among strategies to prepare nitrogen-containing heterocyclic compounds, pathways involving benzyne intermediates are attractive given that they can readily assemble highly diverse heterocyclic compounds in a step-economical manner under transition-metal-free conditions. The synthesis of nitrogen-containing heterocyclic compounds from benzyne intermediates offers an alternative strategy to the conventional metal-catalyzed activation approaches. In the past years, chemists have witnessed the revival of benzyne chemistry, mainly attributed to the wide application of various novel benzyne precursors. The cycloaddition of benzynes is a powerful tool for the synthesis of nitrogen-containing heterocyclic compounds, which can be constructed by [n + 2] cyclization of benzyne intermediates in situ generated from benzyne precursors under mild reaction conditions. This review focuses on the application of cycloaddition reactions involving in situ benzynes in the construction of various nitrogen-containing heterocyclic compounds.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

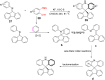
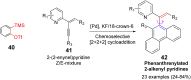
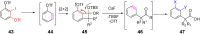
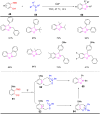
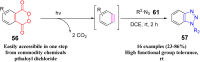
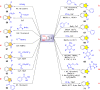
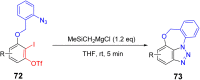
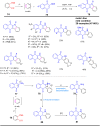
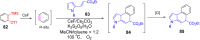
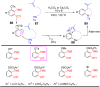
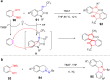


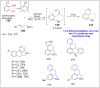
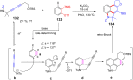

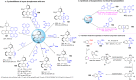
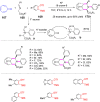
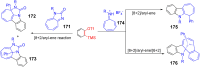


References
-
- Garcia-Lopez J. A. Greaney M. F. Chem. Soc. Rev. 2016;45:6766–6798. - PubMed
-
- Takahashi I. Fujita T. Shoji N. Ichikawa J. Chem. Commun. 2019;55:9267–9270. - PubMed
-
- Hamprecht D. Micheli F. Tedesco G. Checchia A. Donati D. Petrone M. Terreni S. Wood M. Bioorg. Med. Chem. Lett. 2007;17:428–433. - PubMed
-
- Jiaang W.-T. Chen Y.-S. Hsu T. Wu S.-H. Chien C.-H. Chang C.-N. Chang S.-P. Lee S.-J. Chen X. Bioorg. Med. Chem. Lett. 2005;15:687–691. - PubMed
-
- He J. Qiu D. Li Y. Acc. Chem. Res. 2020;53:508–519. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

