A Review of Randomized Controlled Trials of Hereditary Angioedema Long-Term Prophylaxis with C1 Inhibitor Replacement Therapy: Alleviation of Disease Symptoms Is Achievable
- PMID: 36922963
- PMCID: PMC10010185
- DOI: 10.2147/JAA.S396338
A Review of Randomized Controlled Trials of Hereditary Angioedema Long-Term Prophylaxis with C1 Inhibitor Replacement Therapy: Alleviation of Disease Symptoms Is Achievable
Abstract
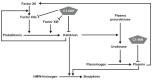
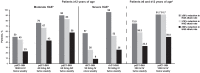
Through its fluctuating disease activity and unpredictable attacks, hereditary angioedema (HAE) imposes a substantial patient burden. To minimize HAE burden and improve quality of life, treatment should involve individualized management strategies that address on-demand therapy and short-term/long-term prophylaxis. Goals of long-term prophylaxis include reducing the number, severity, and burden of HAE attacks. The best characterized forms of HAE arise from deficiency or dysfunction of C1-inhibitor (C1-INH; types I/II), and C1-INH replacement therapy is a first-line intervention for on-demand (acute) treatment of HAE attacks, short-term prophylaxis before high-risk procedures, and long-term prophylaxis. Randomized, double-blind, placebo-controlled crossover trials have shown dose-dependent efficacy with plasma-derived C1-INH (pdC1-INH) 40-60 IU/kg subcutaneously, pdC1-INH 1000 U intravenously, and recombinant human C1-INH (rhC1-INH) 50 IU/kg (maximum 4200 IU) intravenously, all administered twice weekly, as long-term prophylaxis in patients with a history of 2 to ≥4 attacks/month. Overall, up to 83% (pdC1-INH 60 IU/kg) of patients experienced an HAE attack reduction threshold of ≥70%, and up to 58% (pdC1-INH 60 IU/kg) achieved an attack reduction threshold of ≥90%. Lower-dose intravenous pdC1-INH therapy (1000 U) was seemingly less effective, with 45% of 22 patients experiencing an HAE attack reduction threshold of ≥70%, and up to 23% achieving an attack reduction threshold of ≥90%. Higher-dose intravenous rhC1-INH 50 IU/kg (maximum, 4200 IU) twice weekly was of intermediate benefit. Despite a baseline mean attack frequency of 17.9 (during the 3 months prior to study treatment) and a mean attack frequency during a 4-week placebo period of 7.2, 52% of 23 patients experienced ≥70% reduction in attack frequency and 26% of 23 patients experienced ≥90% reduction in attack frequency. The increasing patient percentages treated with C1-INH replacement therapy as long-term prophylaxis meeting these high thresholds reinforces hopes and expectations that "attack freedom" is achievable, including for those with moderate or severe disease.
Keywords: clinical outcomes; clinical trial design; complement C1 inhibitor protein; hereditary angioedema; prophylaxis.
© 2023 Longhurst and Valerieva.
Conflict of interest statement
HL has consulted for, acted as speaker for, or collaborated in research with the following: Adverum, BioCryst, CSL Behring, GSK, Intellia, Ionis, KalVista, Pharming, Pharvaris, and Takeda. She also reports potential research collaboration with Astria. AV has received consultancy/speaker honoraria/meeting sponsorship from, or collaborated in research with, Pharming Group NV, Takeda/Shire, Sobi, CSL Behring, Pharvaris, and Ionis.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

