Mavorixafor, an Orally Bioavailable CXCR4 Antagonist, Increases Immune Cell Infiltration and Inflammatory Status of Tumor Microenvironment in Patients with Melanoma
- PMID: 36923305
- PMCID: PMC10010370
- DOI: 10.1158/2767-9764.CRC-22-0090
Mavorixafor, an Orally Bioavailable CXCR4 Antagonist, Increases Immune Cell Infiltration and Inflammatory Status of Tumor Microenvironment in Patients with Melanoma
Abstract
Purpose: Mavorixafor is an oral, selective inhibitor of the CXCR4 chemokine receptor that modulates immune cell trafficking. A biomarker-driven phase Ib study (NCT02823405) was conducted in 16 patients with melanoma to investigate the hypothesis that mavorixafor favorably modulates immune cell profiles in the tumor microenvironment (TME) and to evaluate the safety of mavorixafor alone and in combination with pembrolizumab.
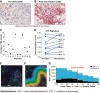
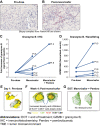
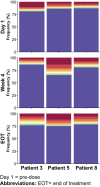
Experimental design: Serial biopsies of melanoma lesions were assessed after 3 weeks of mavorixafor monotherapy and after 6 weeks of combination treatment for immune cell markers by NanoString analysis for gene expression and by multiplexed immunofluorescent staining for in situ protein expression. Serum samples taken at biopsy timepoints were evaluated for key chemokine and cytokine alterations using the Myriad Rules Based Medicine multiplex immunoassays.
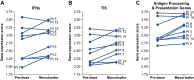
Results: Within the TME, mavorixafor alone increased CD8+ T-cell infiltration, granzyme B signal, antigen presentation machinery, and both tumor inflammatory signature (TIS) and IFNγ gene expression signature scores. Increases in the key serum cytokines CXCL9 and CXCL10 were further enhanced when mavorixafor was combined with pembrolizumab. Adverse events (AE), as assessed by the investigator according to NCI Common Terminology Criteria for Adverse Events (v4.03), related to either mavorixafor or pembrolizumab (≥15%) were diarrhea, fatigue, maculopapular rash, and dry eye. Reported AEs were all ≤ grade 3.
Conclusion/discussion: Treatment with single-agent mavorixafor resulted in enhanced immune cell infiltration and activation in the TME, leading to increases in TIS and IFNγ gene signatures. Mavorixafor as a single agent, and in combination with pembrolizumab, has an acceptable safety profile. These data support further investigation of the use of mavorixafor for patients unresponsive to checkpoint inhibitors.
Significance: Despite survival improvements in patients with melanoma treated with checkpoint inhibitor therapy, a significant unmet medical need exists for therapies that enhance effectiveness. We propose that mavorixafor sensitizes the melanoma tumor microenvironment and enhances the activity of checkpoint inhibitors, and thereby may translate to a promising treatment for broader patient populations.
© 2022 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
R.H.I. Andtbacka reports other from X4 Pharmaceuticals during the conduct of the study. R.H. Pierce reports grants from X4 Pharmaceuticals during the conduct of the study. M. Yushak reports other from X4 Pharmaceuticals during the conduct of the study. M. Milhem reports other from Exicure and Checkmate outside the submitted work. M. Ross reports other from MERCK, MERCK, and AMGEN outside the submitted work. C.C.S. Yeung reports grants from X4 Pharmaceuticals during the conduct of the study; other from Twinstrand, Eli Lilly, Merck, Molpath Dx, Adaptive, and Celegene; grants from Sensei, Signal One, Pfizer, Lonza, Minerva, and OBI outside the submitted work. L.D. Aicher reports grants from X4 Pharmaceuticals during the conduct of the study; grants from X4 Pharmaceuticals outside the submitted work. K.S. Smythe reports grants from X4 Pharmaceuticals during the conduct of the study; personal fees from Spatial Pathology Solutions outside the submitted work. No disclosures were reported by the other authors.
Figures

References
-
- Griffith JW, Sokol CL, Luster AD. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu Rev Immunol 2014;32:659–702. - PubMed
-
- Ratajczak MZ, Zuba-Surma E, Kucia M, Reca R, Wojakowski W, Ratajczak J. The pleiotropic effects of the SDF-1-CXCR4 axis in organogenesis, regeneration and tumorigenesis. Leukemia 2006;20:1915–24. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

