Clinical management of molecular alterations identified by high throughput sequencing in patients with advanced solid tumors in treatment failure: Real-world data from a French hospital
- PMID: 36923436
- PMCID: PMC10009270
- DOI: 10.3389/fonc.2023.1104659
Clinical management of molecular alterations identified by high throughput sequencing in patients with advanced solid tumors in treatment failure: Real-world data from a French hospital
Abstract
Background: In the context of personalized medicine, screening patients to identify targetable molecular alterations is essential for therapeutic decisions such as inclusion in clinical trials, early access to therapies, or compassionate treatment. The objective of this study was to determine the real-world impact of routine incorporation of FoundationOne analysis in cancers with a poor prognosis and limited treatment options, or in those progressing after at least one course of standard therapy.
Methods: A FoundationOneCDx panel for solid tumor or liquid biopsy samples was offered to 204 eligible patients.
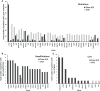
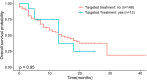
Results: Samples from 150 patients were processed for genomic testing, with a data acquisition success rate of 93%. The analysis identified 2419 gene alterations, with a median of 11 alterations per tumor (range, 0-86). The most common or likely pathogenic variants were on TP53, TERT, PI3KCA, CDKN2A/B, KRAS, CCDN1, FGF19, FGF3, and SMAD4. The median tumor mutation burden was three mutations/Mb (range, 0-117) in 143 patients with available data. Of 150 patients with known or likely pathogenic actionable alterations, 13 (8.6%) received matched targeted therapy. Sixty-nine patients underwent Molecular Tumor Board, which resulted in recommendations in 60 cases. Treatment with genotype-directed therapy had no impact on overall survival (13 months vs. 14 months; p = 0.95; hazard ratio = 1.04 (95% confidence interval, 0.48-2.26)].
Conclusions: This study highlights that an organized center with a Multidisciplinary Molecular Tumor Board and an NGS screening system can obtain satisfactory results comparable with those of large centers for including patients in clinical trials.
Keywords: FoundationOne CDx; cancer; liquid biopsy; next-generation sequencing; precision oncology; targeted therapy.
Copyright © 2023 Pinet, Durand, Perani, Darnaud, Amadjikpe, Yon, Darbas, Vergnenegre, Egenod, Simonneau, Le Brun-Ly, Pestre, Venat, Thuillier, Chaunavel, Duchesne, Fermeaux, Guyot, Lacorre, Bessette, Lalloué, Durand and Deluche.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Targeted Therapy Drug List by Cancer Type - NCI . (2022). Available at: https://www.cancer.gov/about-cancer/treatment/types/targeted-therapies/a... (Accessed September 15, 2022).
-
- Mosele F, Remon J, Mateo J, Westphalen CB, Barlesi F, Lolkema MP, et al. . Recommendations for the use of next-generation sequencing (NGS) for patients with metastatic cancers: A report from the ESMO precision medicine working group. Ann Oncol (2020) 31:1491–505. doi: 10.1016/j.annonc.2020.07.014 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

