TAK-676: A Novel Stimulator of Interferon Genes (STING) Agonist Promoting Durable IFN-dependent Antitumor Immunity in Preclinical Studies
- PMID: 36923556
- PMCID: PMC10010323
- DOI: 10.1158/2767-9764.CRC-21-0161
TAK-676: A Novel Stimulator of Interferon Genes (STING) Agonist Promoting Durable IFN-dependent Antitumor Immunity in Preclinical Studies
Abstract
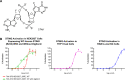
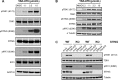
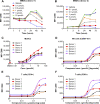
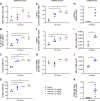
Oncology therapies targeting the immune system have improved patient outcomes across a wide range of tumor types, but resistance due to an inadequate T-cell response in a suppressive tumor microenvironment (TME) remains a significant problem. New therapies that activate an innate immune response and relieve this suppression may be beneficial to overcome this hurdle. TAK-676 is a synthetic novel stimulator of interferon genes (STING) agonist designed for intravenous administration. Here we demonstrate that TAK-676 dose-dependently triggers activation of the STING signaling pathway and activation of type I interferons. Furthermore, we show that TAK-676 is a highly potent modulator of both the innate and adaptive immune system and that it promotes the activation of dendritic cells, natural killer cells, and T cells in preclinical models. In syngeneic murine tumor models in vivo, TAK-676 induces dose-dependent cytokine responses and increases the activation and proliferation of immune cells within the TME and tumor-associated lymphoid tissue. We also demonstrate that TAK-676 dosing results in significant STING-dependent antitumor activity, including complete regressions and durable memory T-cell immunity. We show that TAK-676 is well tolerated, exhibits dose-proportional pharmacokinetics in plasma, and exhibits higher exposure in tumor. The intravenous administration of TAK-676 provides potential treatment benefit in a broad range of tumor types. Further study of TAK-676 in first-in-human phase I trials is ongoing.
Significance: TAK-676 is a novel systemic STING agonist demonstrating robust activation of innate and adaptive immune activity resulting in durable antitumor responses within multiple syngeneic tumor models. Clinical investigation of TAK-676 is ongoing.
Trial registration: ClinicalTrials.gov NCT04879849 NCT04420884 NCT04541108.
© 2022 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
V.A. Appleman reports personal fees from Takeda Development Centers of America, Inc during the conduct of the study; personal fees from Takeda and other from Takeda outside the submitted work. V. Kolev reports other from Takeda during the conduct of the study. M. Mochizuki reports patent application WO2018/100558. S. Haridas reports personal fees from Takeda during the conduct of the study; personal fees from Takeda outside the submitted work. T.D. Thelen reports other from Takeda outside the submitted work. A.O. Abu-Yousif reports other from Takeda during the conduct of the study. M. Okaniwa reports patent application WO2018/100558. No other disclosures were reported.
Figures




References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

