Short-term cognitive loading deteriorates breathing pattern and gas exchange in adult patients with congenital central hypoventilation syndrome
- PMID: 36923564
- PMCID: PMC10009700
- DOI: 10.1183/23120541.00408-2022
Short-term cognitive loading deteriorates breathing pattern and gas exchange in adult patients with congenital central hypoventilation syndrome
Abstract
Question: Human PHOX2B mutations result in life-threatening sleep-related hypoventilation (congenital central hypoventilation syndrome, CCHS). Most patients retain ventilatory activity when awake through a respiratory-related cortical network. We hypothesised that this need to mobilise cortical resources to breathe would lead to breathing-cognition interferences during cognitive loading.
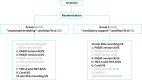
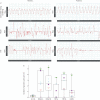
Patients and methods: Seven adult CCHS patients (five women; median age 21) performed standard neuropsychological tests (paced auditory serial addition test - calculation capacity, working memory, sustained and divided attention; trail making test - visuospatial exploration capacity, cognitive processing speed, attentional flexibility; Corsi block-tapping test - visuospatial memory, short-term memory, working memory) during unassisted breathing and under ventilatory support. Ventilatory variables and transcutaneous haemoglobin oxygen saturation were recorded. Cortical connectivity changes between unassisted breathing and ventilatory support were assessed using electroencephalographic recordings (EEG).
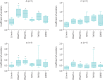
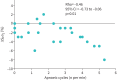
Results: Baseline performances were lower than expected in individuals of this age. During unassisted breathing, cognitive loading coincided with increased breathing variability, and decreases in oxygen saturation inversely correlated with an increasing number of apnoeic cycles per minute (rho -0.46, 95% CI -0.76 to -0.06, p=0.01). During ventilatory support, cognitive tasks did not disrupt breathing pattern and were not associated with decreased oxygen saturation. Ventilatory support was associated with changes in EEG cortical connectivity but not with improved test performances.
Conclusions: Acute cognitive loads induce oxygen desaturation in adult CCHS patients during unassisted breathing, but not under ventilatory support. This justifies considering the use of ventilatory support during mental tasks in CCHS patients to avoid repeated episodes of hypoxia.
Copyright ©The authors 2023.
Conflict of interest statement
Conflict of interest: The study did not involve any conflict of interest, financial or otherwise.
Figures

References
LinkOut - more resources
Full Text Sources
