The effect of the "Oral-Gut" axis on periodontitis in inflammatory bowel disease: A review of microbe and immune mechanism associations
- PMID: 36923589
- PMCID: PMC10008960
- DOI: 10.3389/fcimb.2023.1132420
The effect of the "Oral-Gut" axis on periodontitis in inflammatory bowel disease: A review of microbe and immune mechanism associations
Abstract
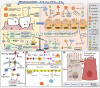
Periodontitis and inflammatory bowel diseases (IBD) are inflammatory diseases of the gastrointestinal tract that share common features of microbial-induced ecological dysregulation and host immune inflammatory response. The close relationship between periodontitis and IBD is characterized by a higher prevalence of IBD in patients with periodontitis and a higher prevalence and severity of periodontitis in patients with IBD, indicating that periodontitis and IBD are different from the traditional independent diseases and form an "Oral-Gut" axis between the two, which affect each other and thus form a vicious circle. However, the specific mechanisms leading to the association between the two are not fully understood. In this article, we describe the interconnection between periodontitis and IBD in terms of microbial pathogenesis and immune dysregulation, including the ectopic colonization of the gut by pathogenic bacteria associated with periodontitis that promotes inflammation in the gut by activating the host immune response, and the alteration of the oral microbiota due to IBD that affects the periodontal inflammatory response. Among the microbial factors, pathogenic bacteria such as Klebsiella, Porphyromonas gingivalis and Fusobacterium nucleatum may act as the microbial bridge between periodontitis and IBD, while among the immune mechanisms, Th17 cell responses and the secreted pro-inflammatory factors IL-1β, IL-6 and TNF-α play a key role in the development of both diseases. This suggests that in future studies, we can look for targets in the "Oral-Gut" axis to control and intervene in periodontal inflammation by regulating periodontal or intestinal flora through immunological methods.
Keywords: IBD; immunity; microbiology; oral-gut axis; periodontitis.
Copyright © 2023 Zhou, Xu, Wang, Jiang, Li, Chao, Sun and A.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Alatab S., Sepanlou S. G., Ikuta K., Vahedi H., Bisignano C., Safiri S., et al. . (2020). The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories 1990–2017: a systematic analysis for the global burden of disease study 2017. Nat. Rev. Gastroenterol. Hepatol. 5, 17–30. doi: 10.1016/s2468-1253(19)30333-4 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

