Method development for simultaneous estimation of Amlodipine Besylate and Perindopril Tertbutyl amine in fixed-dose
- PMID: 36923897
- PMCID: PMC10009730
- DOI: 10.1016/j.heliyon.2023.e14209
Method development for simultaneous estimation of Amlodipine Besylate and Perindopril Tertbutyl amine in fixed-dose
Abstract
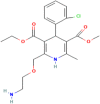
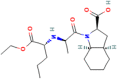
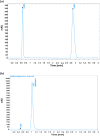
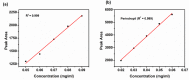
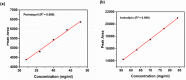
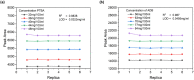
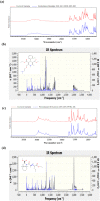
The fixed-dose combination of Amlodipine Besylate (ADB) with Perindopril Tertbutylamine (PTBA) drug is used to treat patients with mild-to-moderate hypertension. In recent times researchers are interested to find the efficient analytical method development and validation for the simultaneous determination of ADB and PTBA in a fixed-dose, film-coated tablet. Therefore, the current study was performed with a reverse-phase liquid chromatography method developed to simultaneously analyze ADB and PTBA in film-coated tablets as fixed-dose combinations. The linearity of the proposed method was calculated by preparing six different mixtures of both ADB and PTBA in the mobile phase. The concentration of both the analytes was analyzed at 56mg/100 mL to 84mg/100 mL and 32mg/100 mL to 48mg/100 mL, respectively. The ratio of acetonitrile and phosphate buffer was 35:65. The flow rate was adjusted to 1.5 ml per minute to reduce the retention time. The validation study was performed for the parameters specificity, linearity, precision, range, limit of detection, limit of quantification, accuracy/biasness, and robustness. The relative percentage standard deviation for Perindopril Tertbutyl amine was 0.148%, and for Amlodipine is 0.312%. These results show that the advanced analysis method for simultaneous analysis of fixed-dose is precise. The theoretical IR spectra were also calculated by Gaussian 9.2 by employing the B3LYP functional at density functional theory (DFT) level study. All these parameters studied in this work authenticate the effectiveness of the developed validation method and ensure its repeatability/reproducibility accordingly. To the best of our knowledge, this is the first time to develop a new fast, and easy method for simultaneous identification and quantification of ADB and PTBA by high-performance liquid chromatography (HPLC) with a time-efficient and cost-effective approach.
Keywords: Amlodipine Besylate (ADB); Fixed-dose; Method development; Perindopril Tertbutylamine (PTBA).
© 2023 The Authors. Published by Elsevier Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Unniachan S., Wu D., Rajagopalan S., Hanson M.E., Fujita K.P. Evaluation of blood pressure reduction response and responder characteristics to fixed-dose combination treatment of amlodipine and losartan: a post hoc analysis of pooled clinical trials. J. Clin. Hypertens. 2014;16:671–677. doi: 10.1111/jch.12390. - DOI - PMC - PubMed
-
- Dennison T.J., Smith J.C., Badhan R.K., Mohammed A.R. Fixed-dose combination orally disintegrating tablets to treat cardiovascular disease: formulation, in vitro characterization and physiologically based pharmacokinetic modeling to assess bioavailability. Drug Des. Dev. Ther. 2017;11:811–826. doi: 10.2147/DDDT.S126035. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

