A Digestive Cartridge Reduces Parenteral Nutrition Dependence and Increases Bowel Growth in a Piglet Short Bowel Model
- PMID: 36924229
- PMCID: PMC10481911
- DOI: 10.1097/SLA.0000000000005839
A Digestive Cartridge Reduces Parenteral Nutrition Dependence and Increases Bowel Growth in a Piglet Short Bowel Model
Abstract
Objective: To determine whether the use of an immobilized lipase cartridge (ILC) to hydrolyze fats in enteral nutrition (EN) reduces parenteral nutrition (PN) dependence in a porcine model of short bowel syndrome with intestinal failure (SBS-IF).
Background: SBS-IF occurs after intestinal loss resulting in malabsorption and PN dependence. Limited therapeutic options are available for achieving enteral autonomy.
Methods: Eleven Yorkshire piglets underwent 75% jejunoileal resection and were randomized into control (n=6) and treatment (n = 5) groups. PN was initiated postoperatively and reduced as EN advanced if predefined clinical criteria were fulfilled. Animals were studied for 14 days and changes in PN/EN calories were assessed. Intestinal adaptation, absorption, and nutrition were evaluated at the end of the study (day 15). Comparisons between groups were performed using analysis of covariance adjusted for baseline.
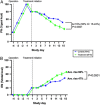
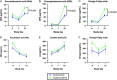
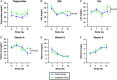
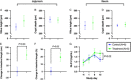
Results: ILC animals demonstrated a 19% greater reduction in PN calories ( P < 0.0001) and higher mean EN advancement (66% vs 47% of total calories, P < 0.0001) during the 14-day experiment. Treatment animals had increased intestinal length (19.5 vs 0.7%, P =0.03) and 1.9-fold higher crypt cell proliferation ( P =0.02) compared with controls. By day 15, ILC treatment resulted in higher plasma concentrations of glucagon-like peptide-2 ( P = 0.02), eicosapentaenoic acid ( P < 0.0001), docosahexaenoic acid ( P = 0.004), vitamin A ( P = 0.02), low-density lipoprotein ( P = 0.02), and high-density lipoprotein ( P = 0.04). There were no differences in liver enzymes or total bilirubin between the two groups.
Conclusions: ILC use in conjunction with enteral feeding reduced PN dependence, improved nutrient absorption, and increased bowel growth in a porcine SBS-IF model. These results support a potential role for the ILC in clinical SBS-IF.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
M.P. and K.M.G. receive research support and advisory compensation from Alcresta Therapeutics, Inc. (Newton, MA). G.L. and E.F. were employees of Alcresta Therapeutics, Inc. at the time of the study. The remaining authors report no conflicts of interest.
Figures

References
-
- Wales PW, de Silva N, Kim J, et al. . Neonatal short bowel syndrome: population-based estimates of incidence and mortality rates. J Pediatr Surg. 2004;39:690–695. - PubMed
-
- O’Keefe SJ, Buchman AL, Fishbein TM, et al. . Short bowel syndrome and intestinal failure: consensus definitions and overview. Clin Gastroenterol Hepatol. 2006;4:6–10. - PubMed
-
- Buchman AL. Etiology and initial management of short bowel syndrome. Gastroenterology. 2006;130(2 suppl 1):S5–S15. - PubMed
-
- Pironi L, Arends J, Baxter J, et al. . ESPEN endorsed recommendations. Definition and classification of intestinal failure in adults. Clin Nutr. 2015;34:171–180. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

