Mechanism and linkage specificities of the dual retaining β-Kdo glycosyltransferase modules of KpsC from bacterial capsule biosynthesis
- PMID: 36924942
- PMCID: PMC10148158
- DOI: 10.1016/j.jbc.2023.104609
Mechanism and linkage specificities of the dual retaining β-Kdo glycosyltransferase modules of KpsC from bacterial capsule biosynthesis
Abstract
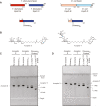
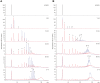
KpsC is a dual-module glycosyltransferase (GT) essential for "group 2" capsular polysaccharide biosynthesis in Escherichia coli and other Gram-negative pathogens. Capsules are vital virulence determinants in high-profile pathogens, making KpsC a viable target for intervention with small-molecule therapeutic inhibitors. Inhibitor development can be facilitated by understanding the mechanism of the target enzyme. Two separate GT modules in KpsC transfer 3-deoxy-β-d-manno-oct-2-ulosonic acid (β-Kdo) from cytidine-5'-monophospho-β-Kdo donor to a glycolipid acceptor. The N-terminal and C-terminal modules add alternating Kdo residues with β-(2→4) and β-(2→7) linkages, respectively, generating a conserved oligosaccharide core that is further glycosylated to produce diverse capsule structures. KpsC is a retaining GT, which retains the donor anomeric carbon stereochemistry. Retaining GTs typically use an SNi (substitution nucleophilic internal return) mechanism, but recent studies with WbbB, a retaining β-Kdo GT distantly related to KpsC, strongly suggest that this enzyme uses an alternative double-displacement mechanism. Based on the formation of covalent adducts with Kdo identified here by mass spectrometry and X-ray crystallography, we determined that catalytically important active site residues are conserved in WbbB and KpsC, suggesting a shared double-displacement mechanism. Additional crystal structures and biochemical experiments revealed the acceptor binding mode of the β-(2→4)-Kdo transferase module and demonstrated that acceptor recognition (and therefore linkage specificity) is conferred solely by the N-terminal α/β domain of each GT module. Finally, an Alphafold model provided insight into organization of the modules and a C-terminal membrane-anchoring region. Altogether, we identified key structural and mechanistic elements providing a foundation for targeting KpsC.
Keywords: 3-deoxy-β-D-manno-oct-2-ulosonic acid; Escherichia coli; KpsC; biosynthesis; capsular polysaccharide; cell surface; enzyme mechanism; enzyme structure; glycolipid; glycosyltransferase.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures








Comment in
-
Trapping and retaining intermediates in glycosyltransferases.J Biol Chem. 2023 Aug;299(8):105006. doi: 10.1016/j.jbc.2023.105006. Epub 2023 Jul 1. J Biol Chem. 2023. PMID: 37394002 Free PMC article.
References
-
- Whitfield C., Wear S.S., Sande C. Assembly of bacterial capsular polysaccharides and exopolysaccharides. Annu. Rev. Microbiol. 2020;74:1–23. - PubMed
-
- Doyle L., Ovchinnikova O.G., Myler K., Mallette E., Huang B.S., Lowary T.L., et al. Biosynthesis of a conserved glycolipid anchor for Gram-negative bacterial capsules. Nat. Chem. Biol. 2019;15:632–640. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

