Time in Brain: How Biological Rhythms Impact on EEG Signals and on EEG-Derived Brain Networks
- PMID: 36925573
- PMCID: PMC10013076
- DOI: 10.3389/fnetp.2021.755016
Time in Brain: How Biological Rhythms Impact on EEG Signals and on EEG-Derived Brain Networks
Abstract
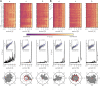
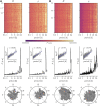
Electroencephalography (EEG) is a widely employed tool for exploring brain dynamics and is used extensively in various domains, ranging from clinical diagnosis via neuroscience, cognitive science, cognitive psychology, psychophysiology, neuromarketing, neurolinguistics, and pharmacology to research on brain computer interfaces. EEG is the only technique that enables the continuous recording of brain dynamics over periods of time that range from a few seconds to hours and days and beyond. When taking long-term recordings, various endogenous and exogenous biological rhythms may impinge on characteristics of EEG signals. While the impact of the circadian rhythm and of ultradian rhythms on spectral characteristics of EEG signals has been investigated for more than half a century, only little is known on how biological rhythms influence characteristics of brain dynamics assessed with modern EEG analysis techniques. At the example of multiday, multichannel non-invasive and invasive EEG recordings, we here discuss the impact of biological rhythms on temporal changes of various characteristics of human brain dynamics: higher-order statistical moments and interaction properties of multichannel EEG signals as well as local and global characteristics of EEG-derived evolving functional brain networks. Our findings emphasize the need to take into account the impact of biological rhythms in order to avoid erroneous statements about brain dynamics and about evolving functional brain networks.
Keywords: biological rhythms; brain dynamics; centrality; clustering coefficient; electroencephalography; functional brain networks; statistical moments; synchronization.
Copyright © 2021 Lehnertz, Rings and Bröhl.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Alsuradi H., Park W., Eid M. (2020). EEG-based Neurohaptics Research: A Literature Review. IEEE Access 8, 49313–49328. 10.1109/access.2020.2979855 - DOI
LinkOut - more resources
Full Text Sources

