Temporal evaluation of efficacy and quality of tissue repair upon laser-activated sealing
- PMID: 36925709
- PMCID: PMC10013809
- DOI: 10.1002/btm2.10412
Temporal evaluation of efficacy and quality of tissue repair upon laser-activated sealing
Abstract
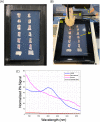
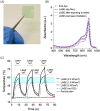
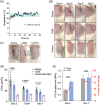
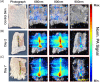
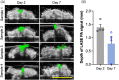
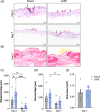
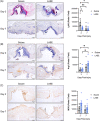
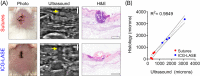
Injuries caused by surgical incisions or traumatic lacerations compromise the structural and functional integrity of skin. Immediate approximation and robust repair of skin are critical to minimize occurrences of dehiscence and infection that can lead to impaired healing and further complication. Light-activated skin sealing has emerged as an alternative to sutures, staples, and superficial adhesives, which do not integrate with tissues and are prone to scarring and infection. Here, we evaluate both shorter- and longer-term efficacy of tissue repair response following laser-activated sealing of full-thickness skin incisions in immunocompetent mice and compare them to the efficacy seen with sutures. Laser-activated sealants (LASEs) in which, indocyanine green was embedded within silk fibroin films, were used to form viscous pastes and applied over wound edges. A hand-held, near-infrared laser was applied over the incision, and conversion of the light energy to heat by the LASE facilitated rapid photothermal sealing of the wound in approximately 1 min. Tissue repair with LASEs was evaluated using functional recovery (transepidermal water loss), biomechanical recovery (tensile strength), tissue visualization (ultrasound [US] and photoacoustic imaging [PAI]), and histology, and compared with that seen in sutures. Our studies indicate that LASEs promoted earlier recovery of barrier and mechanical function of healed skin compared to suture-closed incisions. Visualization of sealed skin using US and PAI indicated integration of the LASE with the tissue. Histological analyses of LASE-sealed skin sections showed reduced neutrophil and increased proresolution macrophages on Days 2 and 7 postclosure of incisions, without an increase in scarring or fibrosis. Together, our studies show that simple fabrication and application methods combined with rapid sealing of wound edges with improved histological outcomes make LASE a promising alternative for management of incisional wounds and lacerations.
Keywords: incisional wounds; laser‐activated sealing; photoacoustic imaging; skin barrier function recovery; tissue adhesive; tissue repair; ultrasound.
© 2022 The Authors. Bioengineering & Translational Medicine published by Wiley Periodicals LLC on behalf of American Institute of Chemical Engineers.
Conflict of interest statement
Kaushal Rege is affiliated with a start‐up company, Synergyan, LLC. Other authors declare no conflict of interest.
Figures
References
-
- MacRae HM, McLeod RS. Handsewn vs. stapled anastomoses in colon and rectal surgery ‐ a meta‐analysis. Dis Colon Rectum. 1998;41(2):180‐189. - PubMed
-
- Beuran M, Chiotoroiu AL, Chilie A, et al. Stapled vs. hand‐sewn colorectal anastomosis in complicated colorectal cancer—a retrospective study. Chirurgia. 2010;105(5):645‐651. - PubMed
-
- Urie R, Flake T, Rege K. Laser tissue welding in wound healing and surgical repair, in bioengineering in wound healing. In: Golberg A, Yarmush ML, eds. Bioengineering in Wound Healing. World Scientific Publishing; 2017:303‐324.
-
- Asencio‐Arana F, Garcia‐Fons V, Torres‐Gil V, et al. Effects of a low‐power He‐Ne‐laser on the healing of experimental colon anastomoses: our experience. Opt En. 1992;31(7):1452‐1457.
-
- Cilesiz I, Springer T, Thomsen S, Welch AJ. Controlled temperature tissue fusion: argon laser welding of canine intestine in vitro. Lasers Surg Med. 1996;18(4):325‐334. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

