Consequences of spinal cord injury on the sympathetic nervous system
- PMID: 36925966
- PMCID: PMC10011113
- DOI: 10.3389/fncel.2023.999253
Consequences of spinal cord injury on the sympathetic nervous system
Abstract
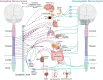
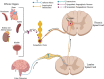
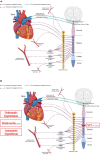
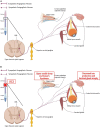
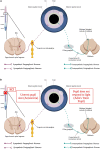
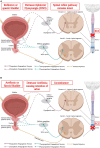
Spinal cord injury (SCI) damages multiple structures at the lesion site, including ascending, descending, and propriospinal axons; interrupting the conduction of information up and down the spinal cord. Additionally, axons associated with the autonomic nervous system that control involuntary physiological functions course through the spinal cord. Moreover, sympathetic, and parasympathetic preganglionic neurons reside in the spinal cord. Thus, depending on the level of an SCI, autonomic function can be greatly impacted by the trauma resulting in dysfunction of various organs. For example, SCI can lead to dysregulation of a variety of organs, such as the pineal gland, the heart and vasculature, lungs, spleen, kidneys, and bladder. Indeed, it is becoming more apparent that many disorders that negatively affect quality-of-life for SCI individuals have a basis in dysregulation of the sympathetic nervous system. Here, we will review how SCI impacts the sympathetic nervous system and how that negatively impacts target organs that receive sympathetic innervation. A deeper understanding of this may offer potential therapeutic insight into how to improve health and quality-of-life for those living with SCI.
Keywords: dysregulation; preganglionic neurons; spinal cord injury; sympathetic innervation; sympathetic nervous system; therapeutics.
Copyright © 2023 Wulf and Tom.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Ackery A., Norenberg M., Krassioukov A. (2007). Calcitonin gene-related peptide immunoreactivity in chronic human spinal cord injury. Spinal Cord 45 678–686. - PubMed
-
- Anderson C. R., McLachlan E. M., Srb-Christie O. (1989). Distribution of sympathetic preganglionic neurons and monoaminergic nerve terminals in the spinal cord of the rat. J. Comp. Neurol. 283 269–284. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

