Utility of laboratory and immune biomarkers in predicting disease progression and mortality among patients with moderate to severe COVID-19 disease at a Philippine tertiary hospital
- PMID: 36926338
- PMCID: PMC10011458
- DOI: 10.3389/fimmu.2023.1123497
Utility of laboratory and immune biomarkers in predicting disease progression and mortality among patients with moderate to severe COVID-19 disease at a Philippine tertiary hospital
Abstract
Purpose: This study was performed to determine the clinical biomarkers and cytokines that may be associated with disease progression and in-hospital mortality in a cohort of hospitalized patients with RT-PCR confirmed moderate to severe COVID-19 infection from October 2020 to September 2021, during the first wave of COVID-19 pandemic before the advent of vaccination.
Patients and methods: Clinical profile was obtained from the medical records. Laboratory parameters (complete blood count [CBC], albumin, LDH, CRP, ferritin, D-dimer, and procalcitonin) and serum concentrations of cytokines (IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-18, IFN-γ, IP-10, TNF-α) were measured on Days 0-3, 4-10, 11-14 and beyond Day 14 from the onset of illness. Regression analysis was done to determine the association of the clinical laboratory biomarkers and cytokines with the primary outcomes of disease progression and mortality. ROC curves were generated to determine the predictive performance of the cytokines.
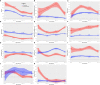
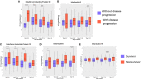
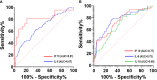
Results: We included 400 hospitalized patients with COVID-19 infection, 69% had severe to critical COVID-19 on admission. Disease progression occurred in 139 (35%) patients, while 18% of the total cohort died (73 out of 400). High D-dimer >1 µg/mL (RR 3.5 95%CI 1.83-6.69), elevated LDH >359.5 U/L (RR 1.85 95%CI 1.05-3.25), lymphopenia (RR 1.91 95%CI 1.14-3.19), and hypoalbuminemia (RR 2.67, 95%CI 1.05-6.78) were significantly associated with disease progression. High D-dimer (RR 3.95, 95%CI 1.62-9.61) and high LDH (RR 5.43, 95%CI 2.39-12.37) were also significantly associated with increased risk of in-hospital mortality. Nonsurvivors had significantly higher IP-10 levels at 0 to 3, 4 to 10, and 11 to 14 days from illness onset (p<0.01), IL-6 levels at 0 to 3 days of illness (p=0.03) and IL-18 levels at days 11-14 of illness (p<0.001) compared to survivors. IP-10 had the best predictive performance for disease progression at days 0-3 (AUC 0.81, 95%CI: 0.68-0.95), followed by IL-6 at 11-14 days of illness (AUC 0.67, 95%CI: 0.61-0.73). IP-10 predicted mortality at 11-14 days of illness (AUC 0.77, 95%CI: 0.70-0.84), and IL-6 beyond 14 days of illness (AUC 0.75, 95%CI: 0.68-0.82).
Conclusion: Elevated D-dimer, elevated LDH, lymphopenia and hypoalbuminemia are prognostic markers of disease progression. High IP-10 and IL-6 within the 14 days of illness herald disease progression. Additionally, elevated D-dimer and LDH, high IP-10, IL-6 and IL-18 were also associated with mortality. Timely utilization of these biomarkers can guide clinical monitoring and management decisions for COVID-19 patients in the Philippines.
Keywords: COVID-19; Filipino; biomarkers; cytokines; disease progression; mortality.
Copyright © 2023 Punzalan, Aherrera, de Paz-Silava, Mondragon, Malundo, Tan, Tantengco, Quebral, Uy, Lintao, Dela Rosa, Mercado, Avenilla, Poblete, Albay, David-Wang and Alejandria.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

