Serum response factor promotes axon regeneration following spinal cord transection injury
- PMID: 36926719
- PMCID: PMC10233768
- DOI: 10.4103/1673-5374.367974
Serum response factor promotes axon regeneration following spinal cord transection injury
Abstract
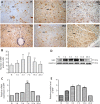
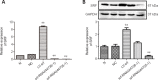
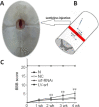
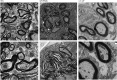
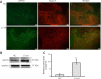
Studies have shown that serum response factor is beneficial for axonal regeneration of peripheral nerves. However, its role after central nervous system injury remains unclear. In this study, we established a rat model of T9-T10 spinal cord transection injury. We found that the expression of serum response factor in injured spinal cord gray matter neurons gradually increased with time, reached its peak on the 7th day, and then gradually decreased. To investigate the role of serum response factor, we used lentivirus vectors to overexpress and silence serum response factor in spinal cord tissue. We found that overexpression of serum response factor promoted motor function recovery in rats with spinal cord injury. Qualitative observation of biotinylated dextran amine anterograde tracing showed that overexpression of serum response factor increased nerve fibers in the injured spinal cord. Additionally, transmission electron microscopy showed that axon and myelin sheath morphology was restored. Silencing serum response factor had the opposite effects of overexpression. These findings suggest that serum response factor plays a role in the recovery of motor function after spinal cord injury. The underlying mechanism may be related to the regulation of axonal regeneration.
Keywords: axon; growth associated protein 43; motor function; myelin sheath; neuron; regeneration; serum response factor; spinal cord; spinal cord transection.
Conflict of interest statement
None
Figures


References
-
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. - PubMed
-
- Curcio M, Bradke F. Axon regeneration in the central nervous system:facing the challenges from the inside. Annu Rev Cell Dev Biol. 2018;34:495–521. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

