Low-temperature 3D-printed collagen/chitosan scaffolds loaded with exosomes derived from neural stem cells pretreated with insulin growth factor-1 enhance neural regeneration after traumatic brain injury
- PMID: 36926724
- PMCID: PMC10233754
- DOI: 10.4103/1673-5374.366497
Low-temperature 3D-printed collagen/chitosan scaffolds loaded with exosomes derived from neural stem cells pretreated with insulin growth factor-1 enhance neural regeneration after traumatic brain injury
Abstract
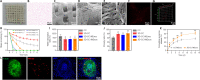
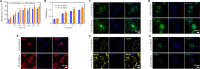
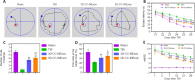
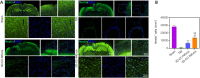
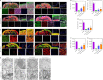
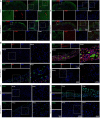
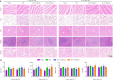
There are various clinical treatments for traumatic brain injury, including surgery, drug therapy, and rehabilitation therapy; however, the therapeutic effects are limited. Scaffolds combined with exosomes represent a promising but challenging method for improving the repair of traumatic brain injury. In this study, we determined the ability of a novel 3D-printed collagen/chitosan scaffold loaded with exosomes derived from neural stem cells pretreated with insulin-like growth factor-1 (3D-CC-INExos) to improve traumatic brain injury repair and functional recovery after traumatic brain injury in rats. Composite scaffolds comprising collagen, chitosan, and exosomes derived from neural stem cells pretreated with insulin-like growth factor-1 (INExos) continuously released exosomes for 2 weeks. Transplantation of 3D-CC-INExos scaffolds significantly improved motor and cognitive functions in a rat traumatic brain injury model, as assessed by the Morris water maze test and modified neurological severity scores. In addition, immunofluorescence staining and transmission electron microscopy showed that 3D-CC-INExos implantation significantly improved the recovery of damaged nerve tissue in the injured area. In conclusion, this study suggests that transplanted 3D-CC-INExos scaffolds might provide a potential strategy for the treatment of traumatic brain injury and lay a solid foundation for clinical translation.
Keywords: 3D printing; angiogenesis; chitosan; collagen; exosomes; functional recovery; insulin-like growth factor-1; neural regeneration; neural stem cells; traumatic brain injury.
Conflict of interest statement
None
Figures


References
-
- Ayer-le Lievre C, Stahlbom PA, Sara VR. Expression of IGF-I and -II mRNA in the brain and craniofacial region of the rat fetus. Development. 1991;111:105–115. - PubMed
-
- Cui L, Saeed Y, Li H, Yang J. Regenerative medicine and traumatic brain injury:from stem cell to cell-free therapeutic strategies. Regen Med. 2022;17:37–53. - PubMed
LinkOut - more resources
Full Text Sources

