Bacteria require phase separation for fitness in the mammalian gut
- PMID: 36927025
- PMCID: PMC10148683
- DOI: 10.1126/science.abn7229
Bacteria require phase separation for fitness in the mammalian gut
Abstract
Therapeutic manipulation of the gut microbiota holds great potential for human health. The mechanisms bacteria use to colonize the gut therefore present valuable targets for clinical intervention. We now report that bacteria use phase separation to enhance fitness in the mammalian gut. We establish that the intrinsically disordered region (IDR) of the broadly and highly conserved transcription termination factor Rho is necessary and sufficient for phase separation in vivo and in vitro in the human commensal Bacteroides thetaiotaomicron. Phase separation increases transcription termination by Rho in an IDR-dependent manner. Moreover, the IDR is critical for gene regulation in the gut. Our findings expose phase separation as vital for host-commensal bacteria interactions and relevant for novel clinical applications.
Conflict of interest statement
Figures

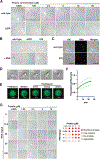
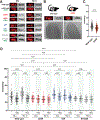

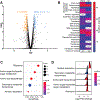
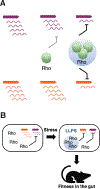
References
-
- Turnbaugh PJ et al., An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444, 1027–1031 (2006). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

