Enhanced Ca2+-Dependent SK-Channel Gating and Membrane Trafficking in Human Atrial Fibrillation
- PMID: 36927079
- PMCID: PMC10147588
- DOI: 10.1161/CIRCRESAHA.122.321858
Enhanced Ca2+-Dependent SK-Channel Gating and Membrane Trafficking in Human Atrial Fibrillation
Abstract
Background: Small-conductance Ca2+-activated K+ (SK)-channel inhibitors have antiarrhythmic effects in animal models of atrial fibrillation (AF), presenting a potential novel antiarrhythmic option. However, the regulation of SK-channels in human atrial cardiomyocytes and its modification in patients with AF are poorly understood and were the object of this study.
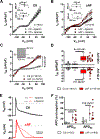
Methods: Apamin-sensitive SK-channel current (ISK) and action potentials were recorded in human right-atrial cardiomyocytes from sinus rhythm control (Ctl) patients or patients with (long-standing persistent) chronic AF (cAF).
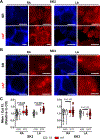
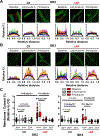
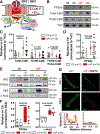
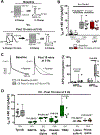
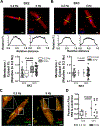
Results: ISK was significantly higher, and apamin caused larger action potential prolongation in cAF- versus Ctl-cardiomyocytes. Sensitivity analyses in an in silico human atrial cardiomyocyte model identified IK1 and ISK as major regulators of repolarization. Increased ISK in cAF was not associated with increases in mRNA/protein levels of SK-channel subunits in either right- or left-atrial tissue homogenates or right-atrial cardiomyocytes, but the abundance of SK2 at the sarcolemma was larger in cAF versus Ctl in both tissue-slices and cardiomyocytes. Latrunculin-A and primaquine (anterograde and retrograde protein-trafficking inhibitors) eliminated the differences in SK2 membrane levels and ISK between Ctl- and cAF-cardiomyocytes. In addition, the phosphatase-inhibitor okadaic acid reduced ISK amplitude and abolished the difference between Ctl- and cAF-cardiomyocytes, indicating that reduced calmodulin-Thr80 phosphorylation due to increased protein phosphatase-2A levels in the SK-channel complex likely contribute to the greater ISK in cAF-cardiomyocytes. Finally, rapid electrical activation (5 Hz, 10 minutes) of Ctl-cardiomyocytes promoted SK2 membrane-localization, increased ISK and reduced action potential duration, effects greatly attenuated by apamin. Latrunculin-A or primaquine prevented the 5-Hz-induced ISK-upregulation.
Conclusions: ISK is upregulated in patients with cAF due to enhanced channel function, mediated by phosphatase-2A-dependent calmodulin-Thr80 dephosphorylation and tachycardia-dependent enhanced trafficking and targeting of SK-channel subunits to the sarcolemma. The observed AF-associated increases in ISK, which promote reentry-stabilizing action potential duration shortening, suggest an important role for SK-channels in AF auto-promotion and provide a rationale for pursuing the antiarrhythmic effects of SK-channel inhibition in humans.
Keywords: actinin; apamin; atrial fibrillation; atrial remodeling; calmodulin; protein phosphatase-2A; protein transport.
Conflict of interest statement
Disclosures
The authors have no relevant conflicts of interest to disclose.
Figures


Comment in
-
Targeting Small-Conductance Calcium-Activated Potassium Channels in Atrial Fibrillation: Therapeutic Opportunities.Circ Res. 2023 Apr 28;132(9):1104-1106. doi: 10.1161/CIRCRESAHA.123.322777. Epub 2023 Apr 27. Circ Res. 2023. PMID: 37104564 Free PMC article. No abstract available.
References
-
- Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42:373–498. doi: 10.1093/eurheartj/ehaa612 - DOI - PubMed

