Assessment of Pre-Clinical Liver Models Based on Their Ability to Predict the Liver-Tropism of Adeno-Associated Virus Vectors
- PMID: 36927149
- PMCID: PMC10150726
- DOI: 10.1089/hum.2022.188
Assessment of Pre-Clinical Liver Models Based on Their Ability to Predict the Liver-Tropism of Adeno-Associated Virus Vectors
Erratum in
-
Correction to: Assessment of Pre-Clinical Liver Models Based on Their Ability to Predict the Liver-Tropism of Adeno-Associated Virus Vectors, by Westhaus et al. Hum Gene Ther 2023;34(7-8):273-288; doi: 10.1089/hum.2022.188.Hum Gene Ther. 2023 Jul;34(13-14):663. doi: 10.1089/hum.2022.188.correx. Epub 2023 May 8. Hum Gene Ther. 2023. PMID: 37155618 Free PMC article. No abstract available.
Abstract
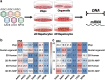
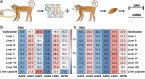
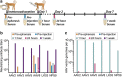
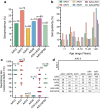
The liver is a prime target for in vivo gene therapies using recombinant adeno-associated viral vectors. Multiple clinical trials have been undertaken for this target in the past 15 years; however, we are still to see market approval of the first liver-targeted adeno-associated virus (AAV)-based gene therapy. Inefficient expression of the therapeutic transgene, vector-induced liver toxicity and capsid, and/or transgene-mediated immune responses reported at high vector doses are the main challenges to date. One of the contributing factors to the insufficient clinical outcomes, despite highly encouraging preclinical data, is the lack of robust, biologically and clinically predictive preclinical models. To this end, this study reports findings of a functional evaluation of 6 AAV vectors in 12 preclinical models of the human liver, with the aim to uncover which combination of models is the most relevant for the identification of AAV capsid variant for safe and efficient transgene delivery to primary human hepatocytes. The results, generated by studies in models ranging from immortalized cells, iPSC-derived and primary hepatocytes, and primary human hepatic organoids to in vivo models, increased our understanding of the strengths and weaknesses of each system. This should allow the development of novel gene therapies targeting the human liver.
Keywords: AAV; hepatocyte; liver gene therapy; nonhuman primate; xenograft model.
Conflict of interest statement
L.L., I.E.A. and A.J.T. have commercial affiliations. L.L. and I.A.E. have consulted on technologies discussed in this article. L.L. and I.A.E. have stock and/or equity in companies with technologies broadly related to this study. L.L. is a co-inventor of, and receives licensing royalties from, several AAV variants used in the study. A.L.M. and T.E.H. are employees, shareholders, and/or optionees of Inventia Life Science Pty. Ltd. Inventia has an interest in commercializing the 3D bioprinting technology. All other authors declare no competing financial interests.
Figures


References
-
- Samulski RJ, Muzyczka N. AAV-mediated gene therapy for research and therapeutic purposes. Annu Rev Virol 2014;1(1):427–451. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
