The efficacy of N-acetylcysteine in chronic obstructive pulmonary disease patients: a meta-analysis
- PMID: 36927162
- PMCID: PMC10026096
- DOI: 10.1177/17534666231158563
The efficacy of N-acetylcysteine in chronic obstructive pulmonary disease patients: a meta-analysis
Abstract
Background: N-acetylcysteine (NAC) may reduce acute exacerbations of chronic obstructive pulmonary disease through an antioxidant effect. Due to the heterogeneity in studies, the currently available data do not confirm the efficacy of oral NAC therapy in chronic obstructive pulmonary disease patients. We hypothesize that chronic obstructive pulmonary disease patients receiving regular oral NAC therapy do not achieve improved clinical outcomes.
Objectives: The purpose of this meta-analysis was to determine the efficacy of long-term oral NAC therapy in chronic obstructive pulmonary disease patients.
Data sources and methods: The literature search was performed using the PubMed, Web of Science, and Cochrane Library databases to identify all included clinical studies. Studies were eligible for inclusion only if they directly compared the outcomes of NAC versus placebo in adults with chronic obstructive pulmonary disease between 1 January 2000 and 30 May 2022. All studies were included if they reported one or more of the following outcomes: number of patients with no acute exacerbations, forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC), St George's Respiratory Questionnaire score, glutathione level, and adverse events.
Results: Nine randomized controlled trials were included in the meta-analysis. There were 1061 patients in the NAC group and 1076 patients in the placebo group. The current meta-analysis provides evidence that the number of patients with no acute exacerbations (965 patients receiving NAC therapy, 979 control group patients), change in FEV1 (433 patients receiving NAC therapy, 447 control group patients), change in FVC (177 patients receiving NAC therapy, 180 control group patients), change in St George's Respiratory Questionnaire score (128 patients receiving NAC therapy, 131 control group patients), change in glutathione levels (38 patients receiving NAC therapy, 40 control group patients), and adverse events (832 patients receiving NAC therapy, 846 control group patients) were not significantly different between the two groups.
Conclusion: NAC did not reduce the risk of acute exacerbation or ameliorate the decline in lung volume in chronic obstructive pulmonary disease patients.
Keywords: N-acetylcysteine; acute exacerbation; chronic obstructive pulmonary disease; forced expiratory volume in 1 s; glutathione.
Conflict of interest statement
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures




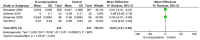




References
-
- Dekhuijzen PN. Antioxidant properties of N-acetylcysteine: their relevance in relation to chronic obstructive pulmonary disease. Eur Respir J 2004; 23: 629–636. - PubMed
-
- Sadowska AM, Manuel-Y-Keenoy B, De Backer WA. Antioxidant and anti-inflammatory efficacy of NAC in the treatment of COPD: discordant in vitro and in vivo dose-effects: a review. Pulm Pharmacol Ther 2007; 20: 9–22. - PubMed
-
- Gul M, Kutay FZ, Temocin S, et al.. Cellular and clinical implications of glutathione. Indian J Exp Biol 2000; 38: 625–634. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

